Olalemi, A. O 1 Atanda, M. O 1 Olawoye, M. A 1Awe, F. T 1 Olalekan, K. O 1 Uchebenu, A. O. 1
1Department of Microbiology, Federal University of Technology, Akure, PMB 704, Ondo State, Nigeria
*Corresponding Author Email: waleolas2002@yahoo.comg …
Highlights
- Water samples were collected from a coastal recreational water.
- Pearson’s correlation was used to evaluate the relationship between microbial and environmental data.
- Levels of nitrate correlated positively with all the opportunistic bacterial pathogens.
- Rainfall had the most significant impact on the levels of the targeted bacteria.
- The risk of infections associated with the pathogens was more significant in children than adults.
Abstract
This study assessed pollution levels, environmental factors, and health risks of enteric bacterial pathogens in tropical coastal water in Araromi, Nigeria. We collected water samples from the beach over 12 months and determined the bacteria load in the samples using the membrane filtration technique. We determined the physicochemical characteristics of the samples using standard methods and measured antecedent rainfall using a weather station close to the beach. We assessed the association between the microbial and environmental data and estimated the risks of infections using dose-response models. Results showed that Salmonella had the highest (3.31 log10 CFU/100 ml) mean value, indicating a potential health risk from Salmonella contamination. In contrast, Clostridium perfringens had the lowest (2.45 log10 CFU/100 ml) mean value in water samples. The mean water temperature was 27.2℃, and the average pH was 7.3. Nitrate content ranged from 2.5 to 12.7 mg/L, whereas rainfall ranged from 21.3 to 385.1 mm, and it had the highest impact on the concentration of enumerated bacteria. The risk of infection with Campylobacter, Salmonella and Shigella were 0.012, 0.0031 and 0.017, respectively, higher than the acceptable risk threshold of 0.0001. Implementing pollution prevention measures, particularly targeting rainfall-induced contamination, is critical to ensure the safety of coastal recreational water.
Keywords: Climate change, faecal pollution, human health, microbial risk, rainfall, recreational water.
1. Introduction
Tropical coastal waters hold significant ecological and economic importance. The water may contain potential hazards due to human activities and climate change. Factors controlling the occurrence of pathogenic organisms in recreational water may include land use, indiscriminate discharge of faecal matter into the water through open defecation, untreated or partially treated sewage and run-off of animal wastes (WHO, 2021). Humans may be exposed to pathogenic microorganisms in water during recreational activities such as swimming and boating. The sources of these pathogens in water are mainly sewage and other diffuse points. Contamination of recreational water by pathogenic organisms may lead to eye, ear, skin, and digestive diseases. Typical examples include listeriosis caused by Listeria monocytogenes and osteomyelitis caused by Staphylococcus aureus. Various bacteria can survive in recreational water; examples of such bacteria are Escherichia coli, Pseudomonas aeruginosa, Klebsiella species, Clostridium perfringens, and Enterococcus faecalis. These enteric and opportunistic bacteria are the major groups of microorganisms responsible for the occurrence of waterborne and water-related diseases. The main transmission pathways are through skin contact and accidental consumption (USEPA, 2012). The microbial quality of recreational water is important to humans who use it for bathing. Poor water quality in tourist-dependent areas may have associated risks of infectious disease outbreaks, leading to reduced economic activities. Therefore, ensuring recreational water safety through routine microbiological assessment of quality is necessary in water resource management for human health protection.
The determination of the presence of a single pathogen in water may provide important information about the pathogen only (Castillo et al., 2015). The microbial quality of water is measured using surrogate or index microorganisms associated with faecal origin of humans or non-humans (Sinclair et al., 2012). Faecal indicator organisms are easy and cheap to detect, and they provide a cost-effective method for assessing the presence of faecal materials in recreational coastal water (Harwood et al., 2017; Nana et al., 2023; Okunade & Olalemi, 2025). There are a few challenges to the use of faecal indicator organisms, this is because they sometimes appear to have no or low predictive value for some enteric pathogens (Boehm et al., 2009; Olalemi et al., 2016; Korajkic et al., 2018).
Excessive rainfall can cause sewers to overflow if they exceed their capacity, and this may lead to beach contamination, closings and aesthetic problems (USEPA, 2022). High temperature, increased rainfall, drought and other extreme climate events have been demonstrated to have a wide impact on the contamination of aquatic environments (Hunter, 2003). The load of microorganisms may reduce or increase depending on the type of the organism and environmental conditions, regardless of the source of pollution (Kolarević et al., 2011). Certain nutrients may alter the physicochemical constituents in water, impacting water quality and usefulness for recreational activities (Caissier, 2006; Kolawole et al., 2011; WHO, 2011; Singh et al., 2013).
The risk of infection due to exposure to a pathogen in water may be determined using Quantitative Microbial Risk Assessment (QMRA). It entails identifying hazards, assessing exposure, dose-response and characterising risk (Olalemi & Akinwumi, 2022; Gholipour et al., 2023; Yang et al., 2023). Activities at the beach in Araromi in Ilaje Local Government Area of Ondo State, Nigeria, are on the increase, and the human population around the area relies on the water not only for recreational purposes but also for fishing and other agricultural and domestic activities. There is limited information on the associated human health risks due to contact or ingestion of the water from the beach and the relationship between rainfall and microbial contamination in the tropical zone. Based on this, the objectives of the study were to determine the load of opportunistic and enteric bacteria in water from Araromi Beach to track changes in water quality, access the physicochemical properties of the samples and antecedent rainfall to determine what linkages exist between the environmental factors and the bacterial isolates; and estimate infection risks due to ingestion of the recreational water.
2. Materials and Methods
2.1 Sampling sites and collection of samples
The study was conducted at the recreational water sampling site situated in Araromi, which lies between longitude 2° 24ˈE˗5° 1ˈE and latitude 5° 51ˈN-6° 42ˈN. Araromi is located in the western part of Ilaje Local Government, Ondo State, Nigeria. The Local Government was carved out from the old Ilaje/Ese-Odo Local Government in 1966. It is bound in the south with the Atlantic Ocean, east by Delta State, north by Okitipupa Local Government and in the west by Ogun State. It has an area of 1,318 km2 and a human population of approximately 450,000. The shoreline covers about 180 km and represents the longest coastline for a State in Nigeria. The sand in the area is mainly made of silt, mud, and superficial sedimentary deposits. Areas on the beach with high anthropogenic activities and proximity to settlements were selected as representative monitoring points. We carried out sampling activities monthly (at 4-week intervals) at the three representative monitoring points over 12 months (i.e., December 2021 to November 2022). Following standard protocol on each sampling occasion, we collected water (10 L) from the beach in sterile plastic bottles. We collected all water samples (n = 72) in duplicates and kept them in cool boxes that contained ice packs. We transported the samples to the laboratory and processed them within 1 h.
2.2 Bacteriological examination of water samples
We determined the concentrations of opportunistic bacterial pathogens, faecal indicator bacteria (FIB) and enteric bacterial pathogens in the samples. We used the membrane filtration technique following standard methods (APHA, 2012). It has a detection limit of 1 cfu/ml. We filtered approximately 100 ml of water through filters of 0.45 μm pore size and thereafter, we placed the filters on selective media (cetrimide agar (CA), membrane faecal coliforms agar (m-FC), membrane lauryl sulphate agar (MLSA), eosin methylene blue (EMB) agar, Salmonella Shigella agar (SSA), Aeromonas-selective agar (ASA), Bifidobacterium-selective agar (BSA), mannitol salt agar (MSA), Listeria-selective agar (LSA), charcoal cefoperozone deoxycholate agar (CCDA) and Clostridium perfringens agar (m-CP). The selective media used had components that enabled the growth and enumeration of the specific bacteria of interest. We incubated agar plates aerobically at 37⁰C for 24 h (ASA, CA, CCDA, MSA, LSA, MLSA, EMB, SSA) and 44⁰C for 24 h (m-FC). We incubated BSA and m-CP anaerobically at 37⁰C for 24 h. Colourless colonies with the black centre were counted as Salmonella, whereas clear, colourless and transparent colonies were counted as Shigella. E. coli had a greenish metallic sheen in the confirmed test, and purple colonies were counted as faecal coliforms; Staphylococcus had yellow colonies; Listeria had cream colonies; Clostridium had yellow, opaque colonies; Aeromonas had green colonies; Bifidobacterium had cream colonies, Campylobacter had a greyish-white metallic sheen, and Pseudomonas had yellow, cream colonies. After incubation, we expressed all colonies as colony-forming units per 100 ml (CFU/100 ml).
2.3 Physicochemical properties of water samples
We determined the temperature of the water samples on-site using a mercury-in-glass thermometer with a measurement accuracy of ±0.01℃ in the range of 0 to 100℃. We measured the salinity, pH, turbidity, electrical conductivity, dissolved oxygen and total dissolved solids using an aquaprobe (PH/ORP/EC/DO, HI98194) with a measurement accuracy of 0.5%FS. Using standard methods, we measured other parameters such as nitrate, biological oxygen demand (BOD), phosphate, chemical oxygen demand (COD), and hardness in the laboratory. In addition, we measured antecedent rainfall using the weather station situated close to the sampling site at Araromi.
2.4 Microbial risk assessment
In this current study, we estimated the risks of infection with enteric bacterial pathogens (Clostridium perfringens, Campylobacter, Salmonella, Shigella) during recreational activities. Clostridium perfringens can produce enterotoxin that may lead to diarrhoea. Ingestion of 106 cells or more may result in an infection. Its short generation time of less than 10 min contributes to its virulence (Melville & Craig, 2013; Choi et al., 2020). Campylobacter causes campylobacteriosis by ingesting 500 or more cells (Medema et al., 1996; Olalemi et al., 2021). Salmonella causes typhoid or non-typhoidal salmonellosis, and ingesting 103 to 105 cells may cause an infection. Shigella causes shigellosis with symptoms such as dysentery from ingesting 10– 100 cells (Crockett et al., 1996). We applied mathematical methods in QMRA to evaluate the risk of infection due to contact or ingestion of opportunistic and enteric bacterial pathogens in water during recreational activities. The beta-Poisson model (Equation 1) was used for exposure to Campylobacter, Salmonella and Shigella, while the exponential model (Equation 2) was used for exposure to Clostridium in water from the beach (Table 1). Furthermore, we determined the annual probability of infection (Equation 3) due to contact or ingestion of water from the beach during recreational activities.
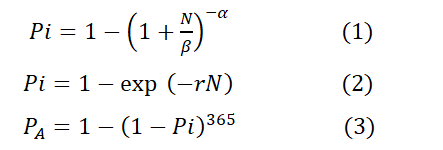
N = exposure (CFU); α, β, r = Parameters determined by the organism’s infectivity; Pi = Probability of infection; PA = Annual probability of infection.
Table 1: Models and parameters adapted to determine risks of infection from opportunistic and enteric bacterial pathogens in water samples from the beach
| Pathogens | Model | Parameters | Reference |
|---|---|---|---|
| Clostridium perfringens | Exponential | r = 1.82 × 10-11 | Choi et al. 2020 |
| Campylobacter | Beta-Poisson | α = 0.145; β = 896 | Medema et al. 1996 |
| Salmonella | Beta-Poisson | α = 0.3126; β = 23,600 | Haas et al. 1999 |
| Shigella | Beta-Poisson | α = 0.2099; β = 1120 | Crockett et al. 1996 |
A single exposure to any of the opportunistic or enteric pathogens may result in some levels of risk to humans. We, therefore, assumed that the consumption of 1 ml, 10 ml and 100 ml during recreational activities was suitable for exposure assessment of pathogens in water from the beach. Specifically, we determined the risk of infection of children and adults to pathogens through ingestion of 37 ml (children) and 16 ml (adults) of water from the beach during recreational activities (Dufour et al., 2006; USEPA, 2011).
2.5 Statistical analysis
We transformed the data obtained to log10 and carried out a one-way analysis of variance (ANOVA), and we separated means using Duncan’s New Multiple Range test on Statistical Package for Social Sciences (SPSS) version 23.0. We used Skewness and kurtosis, Kolmogorov–Smirnov tests to assess the distribution pattern of opportunistic bacterial pathogens, FIB and enteric bacterial pathogens in water from the beach. We subjected all data to correlation analysis to determine the linkages between environmental conditions and the levels of opportunistic bacterial pathogens, FIB and enteric bacterial pathogens in water from the beach.
3. Results and Discussion
3.1 Load of bacteria in water samples
The concentration of faecal coliforms in water samples from the beach ranged from 2.76 to 3.72 log10 CFU/100 ml, E. coli ranged from 2.20 to 2.78 log10 CFU/100 ml, and Bifidobacterium ranged from 1.95 to 2.58 log10 CFU/100 ml.Of the opportunistic bacterial pathogens, Staphylococcus aureus and Pseudomonas aeruginosa had the highest (3.03 log10 CFU/100 ml) and lowest (2.62 log10 CFU/100 ml) mean levels, respectively. Among the enteric bacterial pathogens, Salmonella had the highest (3.31 log10 CFU/100 ml) mean value, whereas Clostridium perfringens had the lowest (2.45 log10 CFU/100 ml) mean value in water samples from the beach (Figure 1). Faecal pollution levels are classified into five categories based on E. coli concentration (log10 CFU 100 ml-1), i.e., Class I – low (≤ 2.0), Class II – moderate (>2.0-3.0), Class III – critical (>3.0-4.0), Class IV – strong (>4.0-5.0) and Class V – excessive (>5.0). E. coli concentration in water from the beach suggests ‘moderate’ faecal contamination. The opportunistic bacterial pathogens, FIB and enteric bacterial pathogens following Kolmogorov–Smirnov tests for normality Ho = 0.05 did not show normal distribution throughout the study (Table 2).
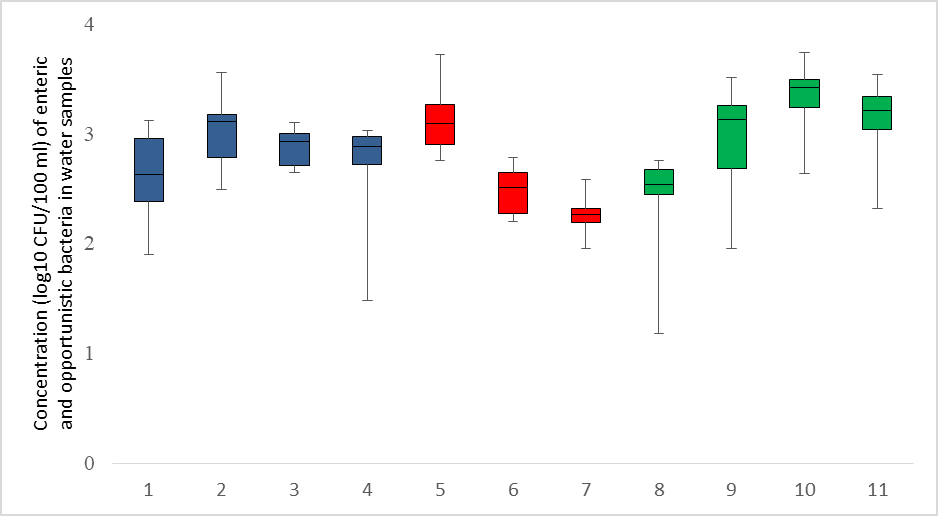
Figure 1: Box plot of meanload of enteric and opportunistic bacteria in water samples from the beach (1 – Pseudomonas aeruginosa; 2 – Staphylococcus aureus; 3 – Aeromonas hydrophilia; 4 – Listeria monocytogenes; 5 – Faecal coliforms; 6 – E. coli; 7 – Bifidobacterium; 8 – Clostridium perfringens; 9 – Campylobacter; 10 – Salmonella; 11 – Shigella).
Table 2: Normality test for bacteria in water samples from the beach
| Microorganisms | Skewness | Kurtosis | Komogorov -Smirnov Statistic |
Normal Distribution |
|---|---|---|---|---|
| Opportunistic Bacterial Pathogens |
||||
| Pseudomonas aeruginosa | -0.354 | -0.607 | 0.614 | No |
| Staphylococcus aureus | -0.029 | -0.697 | 0.873 | No |
| Aeromonas hydrophilia | -0.198 | -1.769 | 0.417 | No |
| Listeria monocytogenes | -1.879 | 2.354 | 0.339 | No |
| Faecal Indicator Bacteria | ||||
| Faecal coliforms | 0.895 | 0.448 | 0.582 | No |
| E. coli | 0.089 | -1.701 | 0.826 | No |
| Bifidobacterium | 0.059 | 1.186 | 0.727 | No |
| Enteric Bacterial Pathogens | ||||
| Clostridium perfringens | -2.810 | 8.744 | 0.527 | No |
| Campylobacter | -0.910 | -0.570 | 0.530 | No |
| Salmonella | -1.218 | 0.884 | 0.577 | No |
| Shigella | -1.395 | 2.012 | 0.714 | No |
3.2 Physicochemical properties of the water samples
The mean water temperature was 27.2℃, and the average pH was 7.3. Values of salinity of the water ranged from 3.9 to 35.7 PSU, and turbidity ranged from 17.2 to 112.6 NTU. The mean value of electrical conductivity of the water was 32508.8 µS/cm, whereas the mean value of total dissolved solids was 16279.5 mg/L. Biological oxygen demand in the water ranged from 46.6 to 215 mg/L, dissolved oxygen values ranged from 3.7 to 8.9 mg/L, and chemical oxygen demand ranged from 87.1 to 417.5 mg/L. Values of nitrate content in the water ranged from 2.5 to 12.7 mg/L, while phosphate values ranged from 9.5 to 16.3 mg/L, suggesting pollution with high nutrient content. Water hardness ranged from 76.0 to 650.0 mg/L. In addition, the average amount of rainfall in the beach area ranged from 21.3 to 385.1 mm (Table 3).
Table 3: Physicochemical properties of the water from the beach and antecedent amount of rainfall
| Physicochemical Parameters | Mean ± SD (Min – Max) |
|---|---|
| Temperature (℃) | 27.2 ± 3.0 (22.3 – 32.0) |
| pH | 7.3 ± 1.4 (5.9 – 11.2) |
| Salinity (PSU) | 21.0 ± 11.3 (3.9 – 35.7) |
| Turbidity (NTU) | 35.0 ± 25.7 (17.2 – 112.6) |
| Total Dissolved Solids (mg/L) | 16279.5 ± 6326.2 (5480 – 23100) |
| Electrical Conductivity (µS/cm) | 32508.8 ± 12644.5 (10900 – 46190) |
| Dissolved Oxygen (mg/L) | 6.0 ± 1.5 (3.7 – 8.9) |
| Biological Oxygen Demand (mg/L) | 142.5 ± 43.2 (46.6 – 215) |
| Chemical Oxygen Demand (mg/L) | 278.9 ± 86.9 (87.1 – 417.5) |
| Nitrate (mg/L) | 8.0 ± 3.5 (2.5 – 12.7) |
| Phosphate (mg/L) | 13.3 ± 2.0 (9.5 – 16.3) |
| Hardness (mg/L) | 439.8 ± 169.7 (76.0 – 650.0) |
| Rainfall (mm) | 167.7 ± 126.5 (21.3 – 385.1) |
Key: Values are expressed as Mean ± Standard Deviation (Minimum – Maximum) (n = 72).
3.3 Relationship between the concentration of bacteria and environmental factors
Pearson’s correlation showed that Pseudomonas aeruginosahad a positive relationship with nitrate (r = 0.70), phosphate (r = 0.55), rainfall (r = 0.91) and a negative relationship with temperature (r = -0.51). Staphylococcus aureus also had a negative relationship with temperature (r = -0.60), while Aeromonas hydrophilia had a positive relationship with nitrate (r = 0.81). Listeria monocytogenes had a positive relationship with nitrate (r = 0.53), rainfall (r = 0.73) and a negative relationship with dissolved oxygen (r = -0.69). Faecal coliforms showed a positive relationship with rainfall (r = 0.69), whereas E. coli exhibited a negative relationship with nitrate (r = -0.73), rainfall (r = -0.53), and Bifidobacterium had a positive relationship with pH (r = 0.72) and a negative relationship with rainfall (r = -0.73). Clostridium perfringens had a positive relationship with nitrate (r = 0.70), rainfall (r = 0.88) and a negative relationship with dissolved oxygen (r = -0.58). Campylobacter had a positive relationship with nitrate (r = 0.67), rainfall (r = 0.90) and a negative relationship with dissolved oxygen (r = -0.64) and temperature (r = -0.54). Salmonella had a positive relationship with nitrate (r = 0.72), rainfall (r = 0.84) and a negative relationship with dissolved oxygen (r = -0.65). Shigella had a positive relationship with nitrate (r = 0.53), rainfall (r = 0.81) and a negative relationship with dissolved oxygen (r = -0.77). In general, antecedent rainfall had the most significant impact on the levels of the targeted bacteria in the water samples from the beach, followed by nitrate content, amount of dissolved oxygen, and then water temperature (Table 4).
Table 4: Correlation between microorganisms, physicochemical properties of water and antecedent rainfall
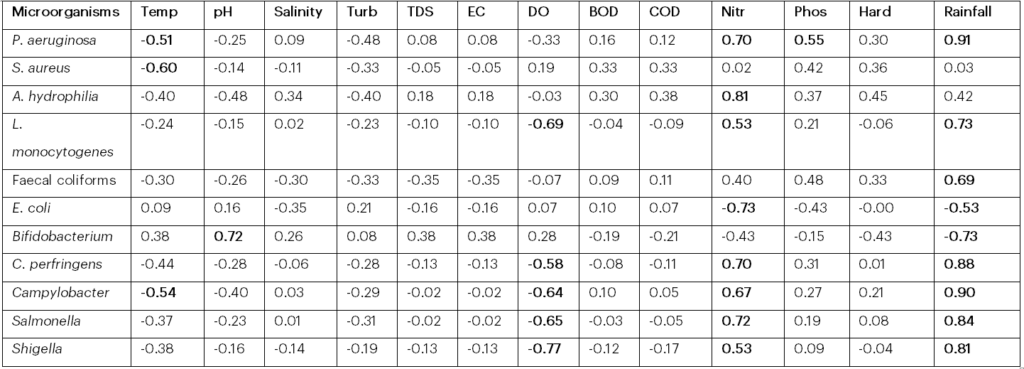
Key: Values in bold indicate correlation; Abbreviations: Temp, temperature; BOD, biological oxygen demand; Turb, turbidity; DO, dissolved oxygen; TDS, total dissolved solids; COD, chemical oxygen demand; EC, electrical conductivity; Nitr, nitrate; Phos, phosphate; Hard, hardness.
3.4 Probability of infection
The probability of infection following contact or ingestion of 100 ml of water from the beach showed that the risk of infection with Campylobacter was 0.12, whereas the risk of infection with Salmonella was 0.031, and the risk of infection with Shigella was 0.17. The probability of infection following contact or ingestion of 10 ml of water from the beach, the risk of infection with Clostridium perfringens was 6.5 × 10-10. Furthermore, the risk of infection with Campylobacter was 0.012, whereas the risk of infection with Salmonella was 0.0031, and the risk of infection with Shigella was 0.017 (Figure 2). The annual probability of infection following contact or ingestion of 100 ml of water from the beach revealed that the annual risk of infection with Clostridium perfringens was 2.4 × 10-6. The annual risk of infection with Campylobacter was 1.0. The annual risk of infection with Salmonella was 1.0, and the annual risk of infection with Shigella was 1.0. The annual probability of infection following contact or ingestion of 10 ml of water from the beach revealed that the annual risk of infection with Clostridium perfringens was 2.4 × 10-7. The annual risk of infection with Campylobacter was 1.0. The annual risk of infection with Salmonella was 0.68, and the annual risk of infection with Shigella was 1.0 (Figure 3).
In children, the probability of infection following contact or ingestion of 37 ml of water from the beach showed that the risk of infection with Clostridium perfringens was 2.4 × 10-9; Campylobacter was 0.044; Salmonella 0.015, while Shigella was 0.063. In adults, the probability of infection following contact or ingestion of 16 ml of water from the beach showed that the risk of infection with Clostridium perfringens was 1.0 × 10-9; Campylobacter was 0.019; Salmonella was 0.005, while Shigella was 0.027 (Figure 4).
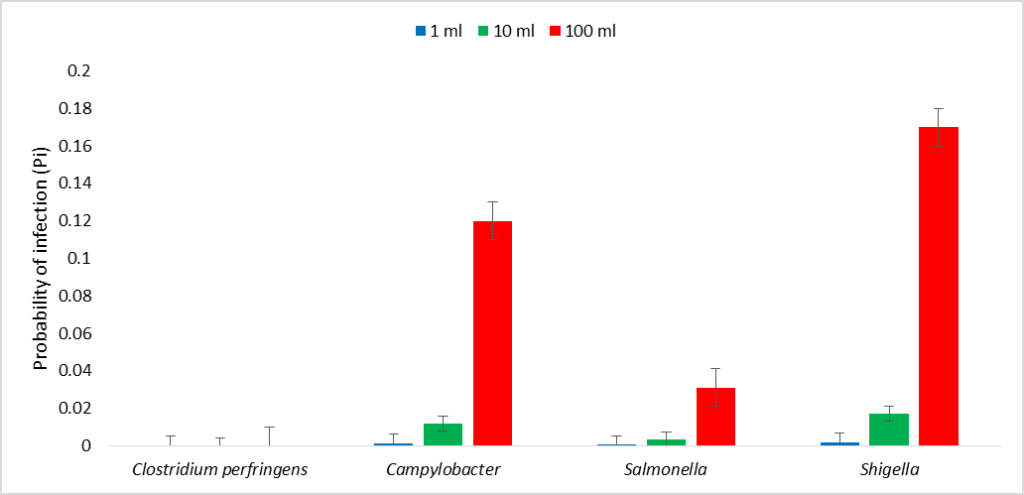
Figure 2: Probability of infection (Pi) due to the pathogens in water from the beach
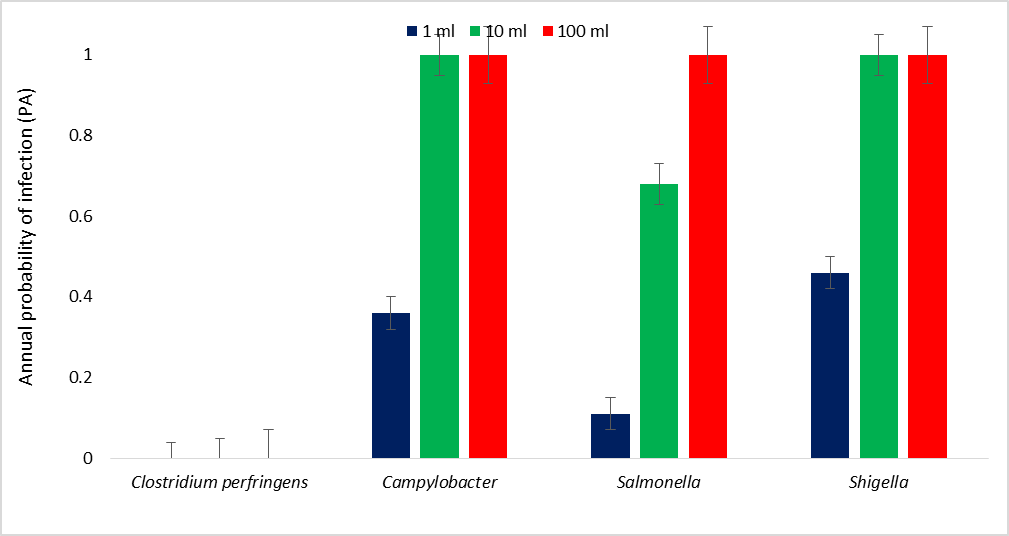
Figure 3: Annual probability of infection (PA) due to the pathogens in water from the beach
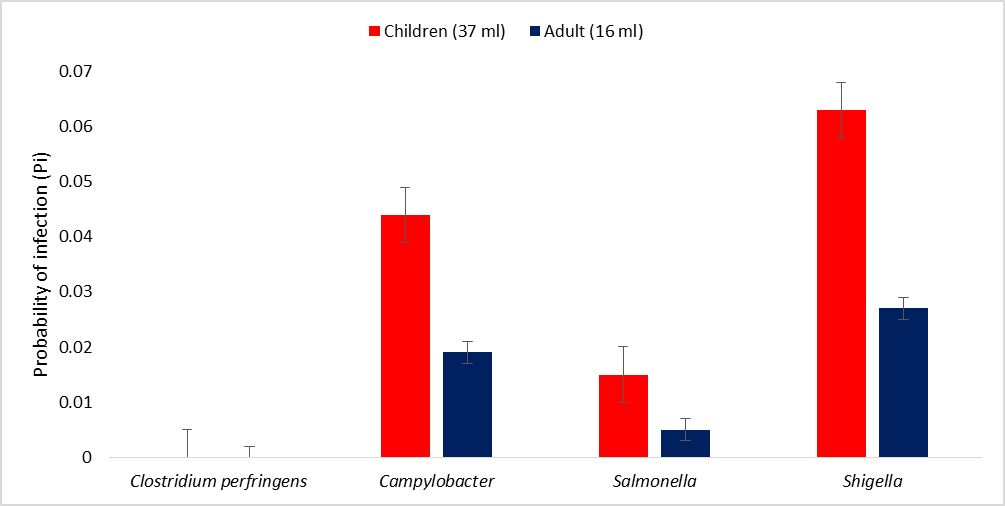
Figure 3: Annual probability of infection (PA) due to the pathogens in water from the beach
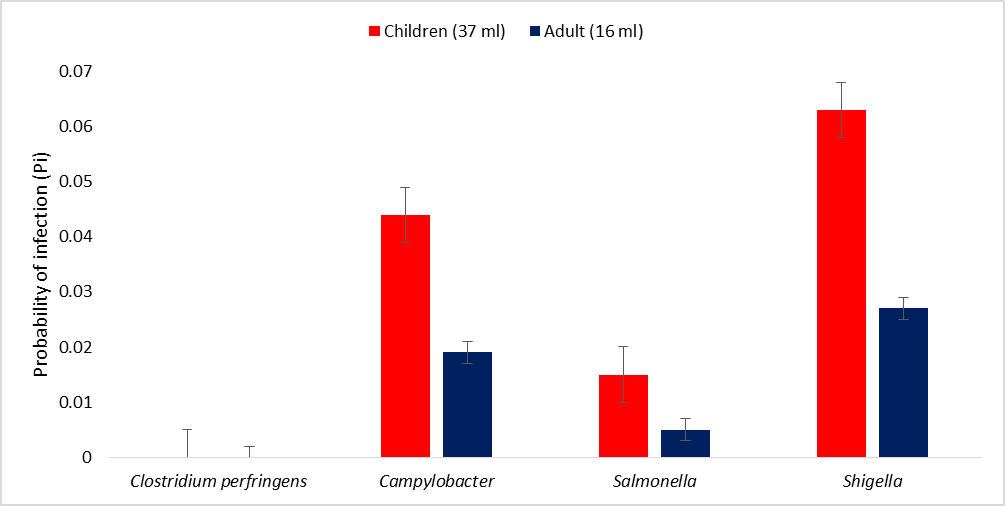
Figure 4: Probability of infection (Pi) of children and adults due to the pathogens in water from the beach (Dufour et al., 2006; USEPA, 2011)
4. Discussion
Recreational water may be contaminated with faecal matter originating from multiple sources (USEPA, 2004). Contamination with sewage may occur from wastewater treatment plants or human facilities for wastewater containment, run-off from urban centres, direct defecation into the water intentionally or accidentally from humans during swimming and bathing activities and contamination from animals such as waterfowl and seagulls. Pseudomonas aeruginosa is an aerobic bacteria found in soil, sewage, water, and skin (USEPA, 2012). It has low persistence and can rapidly die off in aquatic environments; therefore, it is not a suitable indicator for determining faecal pollution. Gholipour et al. (2023) reported that Pseudomonas aeruginosa had the highest occurrence of all opportunistic pathogens detected in coastal water impacted by anthropogenic activities. Similarly, Behera et al. (2023) observed a high load of Pseudomonas aeruginosa during the southwest monsoon in the coastal water of Kakinda, Bengal. In this study, Pseudomonas aeruginosa had the lowest mean concentration of 2.62 log10 CFU/100 ml among the targeted opportunistic bacterial pathogens in water from the beach. This may likely be due to the low persistence of the pathogen in saline water. Staphylococcus aureus is an opportunistic pathogen that infects through tissue invasion (Masalha et al., 2001; Huang et al., 2019). Staphylococcus aureus had the highest mean concentration of3.03 log10 CFU/100 ml among the targeted opportunistic bacterial pathogens in water from the beach. This observation may be due to the high salinity of the water and high anthropogenic activities, such as swimming observed at the representative monitoring points on the beach.
Aeromonas hydrophila is commonly found in fresh, estuarine and marine water in warm climates. Transmission through the faecal-oral route is usually through contact with water (Li et al., 2015; Pal et al., 2016). It may lead to gastroenteritis, especially in children and immunocompromised individuals (Tan et al., 2015; Teunis & Figueras, 2016). It causes two types of gastroenteritis, namely: rice-water diarrhoea (similar to cholera) and dysenteric gastroenteritis (loose stools filled with blood and mucus) (Minnaganti et al., 2000). Aeromonas hydrophila had a high mean concentration of2.88 log10 CFU/100 ml in water from Araromi Beach. This is similar to the findings of Behera et al. (2023), where the authors observed a high load of Aeromonas hydrophila during spring inter-monsoon in the coastal water of Kakinda, Bay of Bengal. Listeria monocytogenes is commonly found in moist environments and is an opportunistic pathogen of humans. Many studies focused on the detection of Listeria monocytogenes in natural environments acting as reservoirs, for example, surface water such as springs and rivers (Schaffter & Parriaux, 2002; Lyautey et al., 2007), natural and artificial ponds (Ferreira et al., 2022), lakes, streams and constructed wetlands (Calheiros et al., 2017). However, there is limited data on the levels and distribution of Listeria monocytogenes in marine environments. This study detected a mean concentration of2.67 log10 CFU/100 ml for Listeria monocytogenes in water from the beach, and this is important in understanding the behavioural dynamics and survival of the pathogen in highly saline aquatic systems. Faecal coliforms are used as indicators of faecal pollution in aquatic systems (Doyle & Erickson, 2006). Faecal coliforms had the highest mean load, 3.12 log10 CFU/100 ml, among the targeted FIB in water from the beach. This observation was above those reported by Nana et al. (2023), where the authors detected levels of faecal coliforms ranging from 5 to 35 CFU/100 ml in water from Kribi beaches along the Southern Atlantic Coast, Cameroon. The mean concentration of faecal coliforms in this study was lower than those reported by Xie et al. (2023), who detected levels of faecal coliforms ranging from 220 to 46,293 CFU/100 ml in a subtropical coastal watershed influenced by faecal contamination from sewage and agricultural run-off. E. coli in water suggest faecal contamination (Doyle & Erickson, 2006). E. coli had a mean load of2.48 log10 CFU/100 ml, and this observation was above those reported by Nana et al. (2023), where the authors detected levels of E. coli that ranged from 0 to 15 CFU/100 ml in water from Kribi beaches along Southern Atlantic Coast, Cameroon. Bifidobacterium is an anaerobic, non-sporulating, Gram-positive rod. It is a potential indicator of faecal contamination in recreational water. Bifidobacterium had the lowest mean concentration of2.26 log10 CFU/100 ml among the targeted FIB in water from the beach. Its presence in water suggests recent contamination because it is unlikely to grow in water due to its physiology and complex growth requirement. Clostridium perfringens is a sulphite-reducing bacteria in the faeces of humans and warm-blooded animals, soil and aquatic environments. Clostridium perfringens had a mean load of2.45 log10 CFU/100 ml in water from the beach. It is persistent in water because of its ability to form spores. Although it is a pathogen of humans, it may also be used to indicate the potential presence of pathogens in water (Melville & Craig, 2013). Campylobacter had the lowest mean concentration of2.19 log10 CFU/100 ml, Salmonella had the highest mean load of3.31 log10 CFU/100 ml, and Shigella had a mean load of3.12 log10 CFU/100 ml among the targeted enteric bacterial pathogens in water from the beach. The presence of these enteric pathogens may pose significant health risks (such as salmonellosis, dysentery, and diarrhoea) to users of the beach in Araromi (Nana et al., 2023; Gao et al., 2024).
Climate change and increasing human impact through anthropogenic activities may contribute to spatiotemporal variation of pollution markers in coastal systems (Xie et al., 2023). The opportunistic bacterial pathogens, FIB and enteric bacterial pathogens that did not have normal distribution throughout the study may be due to pollution originating from multiple sources and the effect of environmental factors on the microorganisms. The mean water temperature of 27.2℃ is an optimum temperature for mesophilic organisms. In general, the pH of seawater may range from 6.0 to 8.5. The mean value of pH 7.3 of water was within the acceptable range. Salinity is used to classify water into fresh, brackish, saline or brine. The salinity of the water from the beach, which ranged from 3.9 to 35.7 PSU, may be classified as brackish or saline. The turbidity of the water, which ranged from 17.2 to 112.6 NTU, indicated the high cloudiness of the water and the potential presence of chemical precipitates and organic and suspended particles. The total dissolved solids in the water was 16279.5 mg/L and can be calculated from electrical conductivity values. The mean electrical conductivity of the water was above 3000 µS/cm and thus unsuitable for irrigational activities (Abbas et al., 2020). Variations in the physicochemical properties of water may be due to nutrient export into the water system. Nutrients play essential roles in aquatic ecosystems by enriching and providing essential components necessary for microbial growth. Nitrate content may be used to categorise water quality into oligotrophic, mesotrophic and eutrophic (Wetzel, 2001; Rustiah et al., 2019). The water from Araromi Beach, with mean values of 8.0 mg/L nitrate content, may be categorised as eutrophic water.
Long-term ecological implications of eutrophication in recreational water may include the generation of offensive odour, development of colour, reduction in dissolved oxygen levels, and release of toxins that may pose significant risks to human and environmental health. Factors that may increase phosphate concentration in water include pollution from industries, markets, and agricultural activities. Phosphate content may be used to classify the richness of water into less rich, average rich, rich and very rich (Rustiah et al., 2019). The water from Araromi Beach, with mean values of 13.3 mg/L phosphate content, is very rich. The average rainfall in the beach area ranged from 21.3 to 385.1 mm. Increasing rainfall may have a wide impact on the pollution of aquatic systems. Antecedent rainfall had the most significant impact on the levels of the targeted bacteria, followed by nitrate content and then the amount of dissolved oxygen. This aligns with findings from other studies on tropical coastal systems (Hunter, 2003; Rustiah et al., 2019; Fern’andez et al., 2020). Behera et al. (2023) observed that increased rainfall increased the load of some bacteria in the coastal water of Kakinda, Bay of Bengal. Export of FIB into a subtropical coastal watershed may be markedly enhanced by high rainfall events and frequent rainfall extremes due to future climate change may likely intensify contamination of the watershed (Fernandez et al., 2020; Xie et al., 2023). Nitrate is one of the most abundant nutrients in aquatic environments. It is an essential nutrient necessary for the growth of microorganisms. The sources of nitrate in water include contamination from sewage, manure, organic fertilisers from agricultural farmland, wastewater from fish farms and soil nitrogen (Testa et al., 2013; Rustiah et al., 2019). The decomposition of dead organic matter by bacteria may also increase nitrate levels in water, which may likely be connected to the observed relationship between bacterial load and nitrate content in recreational water.
QMRA is a valuable tool in risk analysis because it can predict the risk of illness (WHO, 2003; 2005; Yang et al., 2023). The mean risk of infection with Clostridium perfringens was 6.5 × 10-9. Clostridium perfringens is ubiquitous in decaying vegetation, water, soil, faeces, and gastrointestinal tracts of humans and animals. Ingestion of 106 cells or more may result in an infection. Its short generation time of less than 10 min contributes to its virulence (Melville & Craig, 2013; Choi et al., 2020). The mean risk of infection with Campylobacter was 0.12. Campylobacter causes campylobacteriosis. Ingestion of 500 cells or more may lead to an infection in humans (Medema et al., 1996; Olalemi et al., 2021). The mean risk of infection with Salmonella was 0.031. Salmonella causes typhoid or non-typhoidal salmonellosis, and ingesting 103 to 105 cells may cause an infection. The mean risk of infection with Shigella was 0.17. Shigella causes shigellosis, and symptoms such as dysentery and ingesting of 10– 100 cells may lead to an infection (Crockett et al., 1996). The risk of infection with the pathogens followed this order Clostridium perfringens < Salmonella < Campylobacter < Shigella. The estimated risks were all above the USEPA risk limit (10-4), which is acceptable except for Clostridium perfringens. There is an urgent need for targeted interventions to reduce or eliminate the occurrence of these pathogens in the tropical recreational water. In children and adults, infection risks of the pathogens were lowest for Clostridium perfringens and highest for Shigella. Children are generally at greater risk of infection than adults because of their less developed immune systems and greater water volume, likely to be consumed during recreational activities. A key limitation of the study is that the risks of infection associated with dermal and ocular exposures for children and adults were uncertain from the dose-response approach adopted. Further research is therefore recommended in this aspect. This study examined the occurrence of opportunistic bacterial pathogens, FIB and enteric bacterial pathogens in a tropical coastal water used for recreational activities in Araromi, Nigeria. The load of E. coli in water suggests that ‘moderate’ faecal contamination and antecedent rainfall had the greatest impact on levels of the pathogens in the water. The pathogens were not normally distributed in the water, and the risks of infection due to contact or ingestion of the water were all above the risk limit that is acceptable except for Clostridium. We recommend that faecal pollution prevention measures during rainy seasons and routine pathogen detection protocols must be put in place to make tropical coastal water safe for human health. Furthermore, the discharge of untreated or partially treated wastewater into aquatic systems must be discouraged.
5. Conclusion
The detection of specific resistance genes, such as TEM, SHV, NDM, and VIM, across these species underscores the complexity of antibiotic resistance mechanisms and the potential for multidrug resistance, particularly in Vibrio cholerae and Vibrio alginolyticus. The absence of certain resistance genes in Vibrio parahaemolyticus suggests differing evolutionary pressures, indicating that environmental factors play crucial roles in shaping resistance profiles. These findings raise significant concerns regarding food safety and public health, as the presence of these resistant strains in vegetables could pose risks to consumers as well as serve as a reservoir for antibiotic-resistant bacteria. The comprehensive mitigation strategy for addressing Vibrio species antibiotic resistance demands a multifaceted approach encompassing stringent policy interventions, including mandatory molecular screening of food products, implementing national antibiotic stewardship guidelines, regulating agricultural and aquaculture antibiotic usage, developing advanced surveillance systems tracking resistance gene evolution, and promoting alternative treatment modalities such as targeted vaccination protocols and phytotherapeutic interventions, with the ultimate goal of reducing horizontal gene transfer potential, minimising environmental antibiotic exposure, and enhancing molecular diagnostic capabilities to effectively combat the complex ecological dynamics of bacterial adaptation and resistance mechanisms that pose significant public health risks.
6. Acknowledgement
The authors are grateful to the Department of Microbiology, School of Life Sciences, The Federal University of Technology, Akure, Ondo State, Nigeria, for providing appropriate support in terms of equipment and laboratory used for the study.
Funding
The authors declare that no funding was received for the study.
Conflict of Interest
The authors declare no conflict of interest.
References
Abbas, H., Khan, M. Z., Begum, F., Raut, N. & Gurung, S. (2020). Physicochemical Properties of Irrigation Water in Western Himalayas, Pakistan. Water Supply, 20, 3368– 3379.
APHA (2012). Standard Methods for the Examination of Water and Wastewater, 22nd edn. Washington DC, USA: APHA/ AWWA/WEF.
Behera, S., Tanuku, N. R. S., Moturi, S. R. K., Loganathan, J., Modali, S., Tadi, S. R. & Rachuri, V. (2023). Huge Anthropogenic Microbial Load During Southwest Monsoon Season in Coastal Water of Kakinda, Bay of Bengal. Marine Pollution Bulletin, 192: 114977.
Boehm, A. B., Ashbolt, N. J., Colford, J. M., Dunbar, L. E., Fleming, L. E., Gold, M. A., Hansel, J. A., Hunter, P. R., Ichida, A. M., McGee, C. D., Soller, J. A. & Weisberg, S. B. (2009). A Sea Change Ahead for Recreational Water Quality Criteria. Journal of Water and Health, 7 (1): 9–20.
Caissier, D. (2006). Regime of Rivers. A review. Fresh Water Biology, 51: 456–520.
Calheiros, C. S. C., Ferreira, V., Magalhães, R., Teixeira, P. & Castro, P. M. L. (2017). Presence of Microbial Pathogens and Genetic Diversity of Listeria Monocytogenes in a Constructed Wetland System. Ecological Engineering, 102: 344–351.
Castillo, F. Y. R., Muro, A. L., Jacques, M., Garneau, P., González, F. J. A. & Harel, J. (2015) Waterborne Pathogens: Detection Methods and Challenges. Pathogens, 4(2):307–334.
Choi, Y., Kang, J., Lee, Y., Seo, Y., Lee, H., Kim, S., Lee, J., Ha, J., Oh, H., Kim, Y., Byun, K., Ha, S., Yoon, Y. (2020). Quantitative Microbial Risk Assessment for Clostridium perfringens Foodborne Illness Following Consumption of Kimchi in South Korea. Food Science and Biotechnology, http://doi.org/10.1007/s10068-020-00754-2.
Crockett, C. S., Haas, C. N., Fazil, A., Rose, J. B. & Gerba, C. P. (1996). Prevalence of Shigellosis in the US: Consistency with Dose-response Information. International Journal of Food Microbiology, 30, 87– 99.
Doyle, M. P. & Erickson, M. C. (2006). Closing the Door on the Fecal Coliform Assay. Microbe, 1, 162– 163.
Dufour, A. P., Evans, O., Behymer, T. D. & Cantu, R. (2006). Water Ingestion During Swimming Activities in a Pool: A Pilot Study. Journal of Water and Health, 4: 425-430.
Fernandez, A. G., Symonds, E. M., Gallard-Gongora, J. F., Mull, B., Lukasik, J. O., Navarro, P. R., Aguilar, A. B., Peraud, J., Brown, M. L., Alvarado, D. M., Breitbart, M., Cairns, M. R., Harwood, V. J. (2020). Relationships Among Microbial Indicators of Fecal Pollution, Microbial Source Tracking Markers, and Pathogens in Costa Rican Coastal Water. Water Research, 116507.
Ferreira, V., Magalhães, R., Teixeira, P., Castro, P. M. L. & Calheiros, C. S. C. (2022). Occurrence of Fecal Bacteria and Zoonotic Pathogens in Different Water Bodies: Supporting Water Quality Management. Water, 14, 780.
Gao, M., Tan, F., Shen, Y. & Peng, Y. (2024). Rapid Detection Method of Bacterial Pathogens In Surface Waters and a New Risk Indicator for Water Pathogenic Pollution. Scientific Report, 14, 1614.
Gholipour, S., Nikaeen, M., Rabbani, D., Mohammadi, F., Manesh, R. M., Besharatipour, N. & Bina, B. (2023). Occurrence of Enteric and Non-Enteric Microorganisms in Coastal Water Impacted by Anthropogenic Activities: A Multi-Route QMRA for Swimmers. Marine Pollution Bulletin, 114716.
Haas, C. N., Rose, J. B. & Gerba, C. P. (1999). Quantitative Microbial Risk Assessment. New York: Wiley Inc.
Harwood, V. J., Shanks, O., Korajkic, A., Verbyla, M. E., Ahmed, W. & Iriarte, M. (2017). General and Host-Associated Bacterial Indicators of Fecal Pollution. Rose, J. B., Jim énez-Cisneros, B. (Eds.), Global Water Pathogens Project, UNESCO, E. Lans-ing, MI.
Huang, S. S., Singh, R. & McKinnell, J. A. (2019). Decolonisation to Reduce Postdischarge Infection Risk among MRSA Carriers. The New England Journal of Medicine, 380: 638–650.
Hunter, P. R. (2003). Climate Change and Waterborne and Vector-borne Disease. Journal of Applied Microbiology, 94, 37–46.
Kolarević, S., Jelena, K. V. & Momir, P. (2011). Assessment of the Microbiological Quality of the River Tisa in Serbia. Water Research Management, 1, 57-61.
Kolawole, O. M., Ajayi, K. T., Olayemi, A. B. & Okoh, A. I. (2011). Assessment of Water Quality in Asa River (Nigeria) and its Indigenous Clarias Gariepinus Fish. International Journal of Environmental Research and Public Health, 11: 4332-4352.
Korajkic, A., McMinn, B. R. & Harwood, V. J. (2018). Relationships between Microbial Indicators and Pathogens in Recreational Water Settings. International Journal of Environmental Research and Public Health,15 (12), 2842.
Li, F., Wang, W., Zhu, Z., Chen, A., Du, P. & Wang, R. (2015). Distribution, Virulence-associated Genes and Antimicrobial Resistance of Aeromonas Isolates from Diarrheal Patients and Water, China. Journal of Infection, 70: 600–608.
Lyautey, E., Lapen, D. R., Wilkes, G., McCleary, K., Pagotto, F., Tyler, K., Hartmann, A., Piveteau, P., Rieu, A. & Robertson, W. J. (2007). Distribution and Characteristics of Listeria monocytogenes Isolates from Surface Water of the South Nation River Waterhed, Ontario, Canada. Applied and Environmental Microbiology, 73: 5401–5410.
Masalha, M., Borovok, I., Schreiber, R., Aharonowitz, Y. & Cohen, G. (2001). Analysis of Transcription of the Staphylococcus aureus Aerobic Class Ib and Anaerobic Class III Ribonucleotide Reductase Genes in Response to Oxygen. Journal of Bacteriology, 183 (24): 7260–7272.
Medema, G., Teunis, P., Havelaar, A. & Haas, C. (1996). Assessment of the Dose-response Relationship of Campylobacter jejuni. International Journal of Food Microbiology, 30, 101– 112.
Melville, S. & Craig, L. (2013). Type IV pili in Gram-A Positive Bacteria. Microbiol. Mol. Biol. Rev., 77: 323–341.
Minnaganti, V. R., Patel, P. J., Iancu, D., Schoch, P. E. & Cunha, B. A. (2000). Necrotizing Fasciitis caused by Aeromonas hydrophila. Heart & Lung: The Journal of Critical Care. 29 (4): 306–308.
Nana, P. A., Seth, R. E., Tamko, N. A. N., Ossomba, V. R. O., Bricheux, G., Metsopkeng, M. N., Sime-Ngando, T. (2023). Tidal Effect on the Dispersion of Fecal Pollution Indicator Bacteria and Associated Health Risks along the Kribi Beaches (Southern Atlantic Ocean, Cameroon). Regional Studies in Marine Science, 60: 102831.
Okunade, S. O. and Olalemi, A. O. (2025). Widespread Occurrence of Antibiotic Resistance Genes in Bacterial Isolates from River Ala in Akure, Nigeria. South Asian Journal of Research in Microbiology, 19(1): 1-18.
Olalemi, A. O., Ige, O. M., James, G. A., Obasoro, I. F., Okoko, F. O. & Ogunleye, C. O. (2021). Detection of Enteric Bacteria in Two Groundwater Sources and Associated Microbial Health Risks. Journal of Water and Health, 19, 322– 335.
Olalemi, A., Baker-Austin, C., Ebdon, J. & Taylor, H. (2016). Bioaccumulation and Persistence of Faecal Bacterial and Viral Indicators in Mytilus edulis and Crassostrea gigas. International Journal of Hygiene and Environmental Health, 219, 592– 598.
Olalemi, A. O. & Akinwumi, I. M. (2022). Microbial Health Risks Associated with Rotavirus and Enteric Bacteria in River Ala in Akure, Nigeria. Journal of Applied Microbiology, 5, 3995–4006.
Pal, B. B., Pattnaik, S. K., Mohanty, A., Samal, S. K., Khuntia, H. K. & Nayak, S. K. (2016). Incidence of Aeromonas species Isolated from Diarrhoea Patients and Water Samples from Coastal Districts of Odisha, India. International Journal of Current Microbiology and Applied Science, 5, 990–999.
Rustiah, W., Noor, A., Maming, L., Baharuddin, M. & Fitriyah, A. T. (2019). Distribution Analysis of Nitrate and Phosphate in Coastal Area: Evidence from Pangkep River, South Sulawesi. International Journal of Agriculture System, 7(1): 9-17.
Schaffter, N. & Parriaux, A. (2002). Pathogenic-bacterial Water Contamination in Mountainous Catchments. Water Res., 36: 131–139.
Sinclair, R. G., Rose, J. B., Hashsham, S. A., Gerba, C. P. & Haas, C. N. (2012). Criteria for Selection of Surrogates Used to Study the Fate and Control of Pathogens in the Environment. Applied and Environmental Microbiology, 78, 1969–1977.
Singh, K., Singh, B. & Singh, R. R. (2013). Effect of Land Rehabilitation on Physicochemical and Microbial Properties of a Sodic Soil. Catena, 109: 49–57.
Tan, B., Ng, C., Nshimyimana, J. P., Loh, L. L., Gin, K. Y-H. & Thompson, J. R. (2015). Next-Generation Sequencing (NGS) for Assessment of Microbial Water Quality: Current Progress, Challenges, and Future Opportunities. Frontiers in Microbiology, 6, (1027): 1–20.
Testa, J. M., Brady, D. C., Di Toro, D. M., Boynton, W. R. & Kemp, W. M. (2013). Sediment Flux Modeling: Nitrogen, Phosphorus and Silica Cycles. Estuarine, Coastal and Shelf Science. http://doi.org/10.1016/j.ecss.2013.06.014
Teunis, P. & Figueras, M. J. (2016). Reassessment of the Enteropathogenicity of Mesophilic Aeromonas species. Frontiers in Microbiology, 7:1395.
USEPA (2004). Water Quality Standards for Coastal and Great Lakes Recreation Water Rule (BEACH Act). 69 FR 67217. U.S. Environmental Protection Agency, Washington, DC.
USEPA (2011). Exposure Factors Handbook. 2011 Edition. National Centre for Environmental Assessment, Washington D.C; EPA/600/R-09/052F.
USEPA (2012). Recreational Water Quality Criteria. Publication No. EPA 820-F-12-058. U.S. Environmental Protection Agency, Washington, DC.
USEPA (2022). EPA’s Beach Report: 2021 Swimming Season. Office of Water EPA 823-R-22-002. U.S. Environmental Protection Agency, Washington, DC.
Wetzel, R. G. (2001). Limnology Lake and River Ecosystem. 3th Ed. Academica Press. San Diego, California.
WHO (2003). Emerging Issues in Water and Infectious Diseases. Geneva: World Health Organization.
WHO (2011). Technical Guidance on Water-Related Disease Surveillance. Geneva: World Health Organization.
WHO (2021). Guidelines on Recreational Water Quality, Volume 1: Coastal and Fresh Water. Geneva: World Health Organization.
Xie, Z., Chen, S., Huang, J., Li, D. & Lu, X. (2023). Patterns and Drivers of Fecal Coliform Exports in a Typhoon-Affected Watershed: Insights from 10-Year Observations and SWAT Model. Journal of Cleaner Production, 406: 137044.
Yang, Y., Deng, Y., Shi, X., Liu, L., Yin, X., Zhao, W., Li, S., Yang, C. & Zhang, T. (2023). QMRA of Beach Water by Nanopore Sequencing-Based Viability-Metagenomics Absolute Quantification. Water Research, 119858.
About this Article
Cite this Article
APA
Olalemi, A. O., Atanda, M. O., Olawoye, M. A., Awe, F. T., Olalekan, K. O. & Uchebenu, A. O.(2025). Antecedent Rainfall Impacted Levels of Enteric Bacteria and Opportunistic Bacterial Pathogens in Coastal Recreational Water in Araromi, Nigeria. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Olalemi, A. O., Atanda, M. O., Olawoye, M. A., Awe, F. T., Olalekan, K. O. and Uchebenu, A. O. 2025. “Antecedent Rainfall Impacted Levels of Enteric Bacteria and Opportunistic Bacterial Pathogens in Coastal Recreational Water in Araromi, Nigeria” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
20 November 2024
Accepted
10 January 2025
Published
4 February 2025
Corresponding Author Email: waleolas2002@yahoo.com
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.