Akele, O. E. 1 Osazuwa C. O. 2 Kelly B. A. 3 Adejuigbe, A. 4 & Oyewale, M. B. R. 5
1Department of Microbiology, Adekunle Ajasin University Akungba-Akoko, 342111, Nigeria.
2Department of Animal and Environmental Biology, Adekunle Ajasin University, 342111, Nigeria.
3Department of Biochemistry, Adekunle Ajasin University Akungba-Akoko, 342111, Nigeria.
*Corresponding Author Email: emmkel2005@yahoo.com …
Abstract
Ethanol, N-hexane and water extracts of two selected local herbal plants, Eleusine indica and Carica papaya roots, were screened for their antimicrobial activities against some multiple drug-resistant clinical isolates. The solvents were selected due to their difference in polarity to maximize the phytochemicals that will be extracted. Bacillus subtillis, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumonia and Shigella dysentriae using Agar well diffusion standard method. A standard antibiotic, Tetracycline and Chloramphenicol, were used as positive control due to their wide spectrum ability with varied zones of inhibition ranging from (16mm to 31mm). Ethanol, N-hexane and water extract from both plants were selectively active against all the test organisms which justified the use of the herbal plants in treating diarrhoea, wound ulcer and other infections by the locals. The ethanol extracts had the Lowest minimum inhibitory concentration (MIC) (12.5mg/ml to 100mg/ml) (25mg/ml to 100mg/ml) than water extract (75mg/ml to 100mg/ml). (MBC) (75mg/ml and 100mg/ml) had a bactericidal effect on test organisms. The results obtained in the study suggest that the selected plants could be suitable
Keywords: Antimicrobial activity, local plants, pathogenic organisms, Eleusine indica, herbal medicine.
1. Introduction
Microorganisms have evidently existed since life on earth began (Awake, 2003). These organisms have consistently shown to be resistant against many existing antimicrobial drugs (Awake, 2003). Okigbo et al. (2009) and Obire et al. (2017) discussed that the occurrence of microbial resistance to existing antimicrobial drugs highlights the need for the incessant search for new antimicrobials. One of the opportunities for such a quest is to screen local medicinal plants for likely antimicrobial activities (Okigbo & Omodamiro, 2004); (Ahmed et al., 2014).
In an attempt to win the battle against infectious diseases, new antibiotics have been regularly introduced into medicine from the 1910s through the early 2000s to treat bacteria that defied earlier drugs. However, within a few years, strains of bacteria surfaced that defied the so-called new drugs as well, and humans have come to learn that bacterial resistance will remain a continual challenge (Awake, 2003). As the use of antibiotics has increased, resistant strains of bacteria have multiplied and spread. This has increased substantially in recent years due to the indiscriminate use of antibiotic drugs (Casey et al., 2023) and is posing an ever-increasing therapeutic problem in the treatment of infectious diseases (Suaifan et al., 2012).
Ahmed et al. (2014) stated that plants may offer a new source of antibacterial agents as they contain numerous biologically active compounds which may have antimicrobial properties (Cowan, 1999 and Kurek et al., 2012). Plant metabolites are also known to disrupt bacterial cell walls, membranes, and nucleic acid synthesis, reducing the likelihood of resistance development. They equally possess the ability to disrupt biofilms, which are protective structures that contribute to bacterial resistance. (Burt, 2004). Plant-derived medicines have been part of traditional health care in most parts of the world for thousands of years, and there has been increasing interest in plants as sources of agents to fight microbial diseases. The use of antibiotic inhibitors of plant origin is now advocated as one of the strategies to reduce their resistance to antibiotics and other adverse effects on humans (Kumar et al., 2018).
The present study was conducted to evaluate the antimicrobial activities of two local herbal plants, Stubborn grass (Eleusine indica) and Pawpaw (Carica papaya roots), traditionally used in Akoko communities in Ondo State with a view to determining their potency on selected microbial pathogens due to earlier reports about their antimicrobial potentials (Dagne et al., 2021); (Cosca et al., 2019).
2. Materials and Methods
2.1 Collection and preparation of samples
The herbal plants Stubborn grass (Eleusine indica) and Pawpaw (Carica papaya roots) were collected from the Akungba Akoko area of Ondo State, Nigeria, during the rainy season between June and August. The specimens were deposited at the Herbarium of the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba Akoko for confirmation of identities by the taxonomists. The laboratory work was done in the microbiology laboratory at Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria.
2.2 Sources of test microorganisms
Pure cultures of the test organisms (Bacillus subtillis, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumonia, and Shigella dysentriae) were obtained from the microbiology unit of the laboratory in Federal Medical Center, Owo, Ondo State, Nigeria. No ethical approval was needed to access the isolates.
2.3 Extraction of plant materials
Healthy whole leaves of both plants, including stubborn grass (Eleusine indica) and Pawpaw (Carica papaya roots), were washed with sterile water. They were cut into thin slices and rinsed several times with sterile water. The clean slices were spread on sterile trays and dried in a shed. The dried samples were powdered using a laboratory milling machine, as reported by Okigbo et al. (2009).
2.4 Ethanol extraction
A 50g portion of each identified plant (Eleusine indica and Carica papaya roots) was pulverized and soaked in 150 ml of distilled water, N-hexane, and ethanol, respectively, for 72 hours, with constant agitation of the mixtures in a shaker water bath (DKZ-2 Series, T1208221) at room temperature. The extract was then filtered using Whatman no. 1 filter paper and dried with the aid of a Rotary Evaporator RE52-2 Search Tech. Instruments (Okigbo et al., 2009).
2.5 Preparation of Standard Liquid Media for the Growth of Fungi and Bacteria
Thirteen 13 g of nutrient broth powder was dissolved in 1 L of water, while 30 g of Sabouraud dextrose agar was dissolved into 1 L of water as standard liquid media for culturing bacteria and fungi, respectively (Fawole & Oso, 2001). They were dispensed into smaller conicals, and the resulting media were sterilized at 121 C for 15 min. Media were allowed to cool after sterilization before inoculation with the selected microbe. For the standard solidified media, 28 g of Nutrient agar and 65 g of Sabouraud dextrose agar were dissolved in 1 L of water, sterilized and allowed to cool, respectively.
2.6 Inoculation of Formulated Media with Microorganisms
The bacteria strains were cultured for 24 hours at 37 OC in sterile Nutrient broth. Each inoculum was then serially diluted using sterile physiological saline to match approximately 0.5 McFarland standards [ca 108 cfu/ml] (NCCLS, 1990). Plates containing Mueller Hinton agar were dried and then flooded with standardized inoculums of the clinical isolates as described by (Atata et al., 2013); sterile cork borer was used to create wells of 8mm in size, and the bottom of the wells were partly covered with molten agar to prevent the plant extract solution from draining away pasty reconstituted different plant extract was added into the well, allowed to diffuse for 1 hour then incubated at 37 OC for 18 to 24 hours. Controls were set up using standard antibiotics. The diameters of the zone of inhibition were measured and recorded in millimetres.
2.7 Determination of Minimum Inhibitory Concentration (MIC) of the Isolates
The minimum inhibitory concentration (MIC) of the extracts was carried out to assess the lowest concentration of each extract that could inhibit the test organisms. MIC was determined using the macro broth serial dilution technique (Lennette et al., 1990; Bary, 1976). Stock solutions of the plant extracts were prepared. Serial dilutions were carried out from stock solution to give a series of concentrations of 0.5mg/ml, 1.0mg/ml, 1.5mg/ml, 2.0mg/ml and 2.5mg/ml to determine the least effective concentration. Standardized overnight cultures of the isolates were then inoculated into the tubes and incubated at 37oC for 24 hrs. Positive and negative controls were set up. After incubation, the tubes were observed for growth. Based on the pattern of growth in the tubes, the MIC of the extract was observed and recorded in millimetres.
3. Results
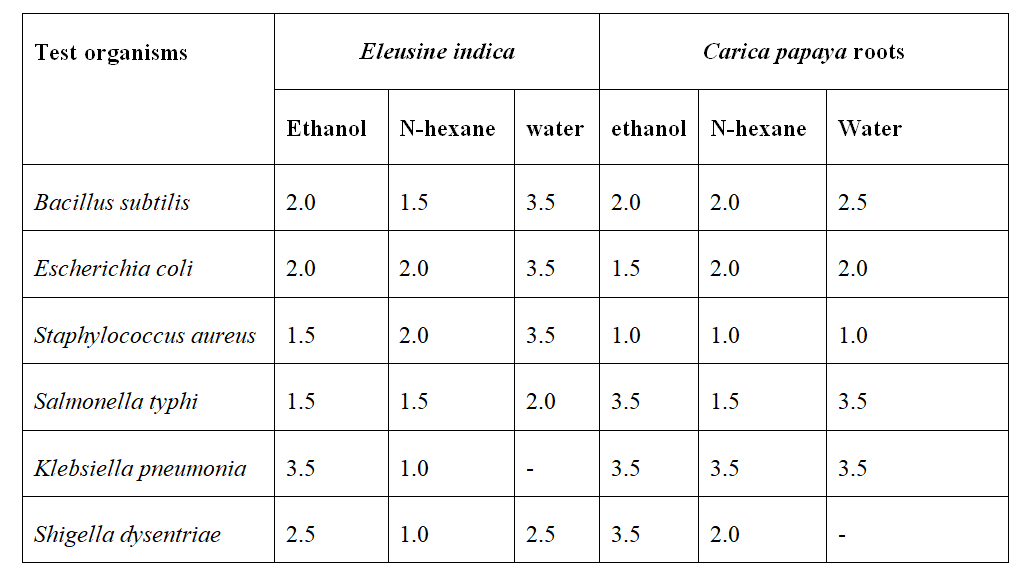
Table 1: Zone of inhibition in mm exhibited by ethanol, N-hexane and water extracts of test plants

Key: Sensitive 15mm ≥, intermediate 14mm -10mm and 9≤ resistant
Table 2: MIC values (mg/ml) for ethanol and water extracts of stubborn grass (Eleusine indica) and Pawpaw (Carica papaya roots).
4. Discussion
In this study, the antimicrobial activities of Eleusine indica and Carica papaya were examined. The ethanol and N-hexane extract of both plants examined had wider clear zones surrounding them where the test organisms were unable to grow compared to the zones surrounding the water extract. This may be because ethanol and n-hexane are organic solvents and extract organic compounds better than water, as reported by (Okigbo et al., 2016; and Kurek et al., 2012). The result of this study shows that the bioactive components of Eleusine indica and Carica papaya roots have bacteriocidal and bacteriostatic activities, which support their traditional use in various parts of Africa for the treatment of various stubborn infections by traditionalists (Okigbo et al., 2009; Casey et al., 2023). All Gram-positive (Bacillus subtilis andStaphylococcus aureus) and Gram-negative bacteria (Escherichia coli, Salmonella typhi, Klebsiella pneumonia, Shigella dysentriae) examined in this study were inhibited to different degrees. The choice of test organisms used in this study is due to their ability to often cause human diseases such as common colds, wounds, sores, sexually transmitted infections, meningitis, respiratory, urinary and respiratory symptoms (Waihenya et al., 2002).
The result of the MIC supports the findings of Ibeh et al. (2002), Okigbo and Omadamiro (2004), and Okigbo et al. (2009) that zones of inhibition reduce as the extract concentration was diluted with water hence, reducing the bactericidal ability of the extract. The study also shows that the control drug with the highest zone of inhibition is chloramphenicol against Salmonella typhi with a size of 11 mm, which is smaller compared to the zones of inhibition obtained from our crude extracts, with zones of inhibition reaching as high as 20 mm in the case of crude n-hexane extract of Eleusine indica against Klebsiella pneumoniae thus suggesting that our extracts is even more potent compared to the standard drugs against some organisms.
5. Conclusion
This study shows that Eleusine indica and Carica papaya have enormous therapeutic potential and can be employed, especially by traditional healers. They can also be further processed into standard drugs for use against infectious agents. Typical of many natural products, they assure of much lesser side effects often associated with synthetic antimicrobials.
6. Acknowledgement
The authors would like to express their appreciation for the efforts of Swezey James of the Agricultural Research Service Culture Collection Unit, United States Department of Agriculture, in providing the entomopathogenic cultures used in this study. The provision of these cultures not only facilitated key experiments but also enriched the scope of this study, allowing for deeper insights into the biological control of insect pests.
Conflict of Interest
The was no conflict of interest during this study.
References
Ahmed, A., Wang, Y., & Qin, G. (2014). Effect of green tea polyphenol on Pseudomonas aeruginosa biofilm formation and quorum sensing. Science Letters, 5(3), 124–131.
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology, 94(3), 223–253.
Casey, J. A., Tartof, S. Y., Davis, M. F., Nachman, K. E., Price, L., Liu, C. & Rudolph, K. E. (2023). Impact of a state-wide livestock antibiotic use policy on resistance in human urine Escherichia coli isolates: a synthetic control analysis. Environmental Health Perspectives, 131(2), 027007.
Cosca, R. T., Santos, S. P., Agabe, J. E., Fernandez, A. S., & Ruiz, A. A. (2019). Antibacterial property of powdered root of eleusine indica (goosegrass) extract against Escherichia coli and Staphylococcus aureus. [Research output, De La Salle Medical and Health Sciences Institute]. GreenPrints. https://greenprints.dlshsi.edu.ph/grade_12/11/
Dagne E, Dobo B, Bedewi Z. (2021) Antibacterial Activity of Papaya (Carica papaya) Leaf and Seed Extracts Against Some Selected Gram-Positive and Gram- Negative Bacteria. Pharmacognosy Journal. 13(6s):1727-1733.BibTex XML PDF (1.04 MB)
Ibeh, J. N., Idu, M., & Obasuyi, O. (2002). Studies on the antimicrobial properties ofLepistemuno wariense leaf. Journal of Medical Laboratory Service, 11:40-45.
Kumar, M., Sarmah, A. K., & Pannu, J. (2018). “Emerging green chemical technologies for sustainable antibiotics suppression and treatment: A systematic review.” Journal of Hazardous Materials, 357, 280-295.
Kurek, A., Nadkowska, P., Pliszka, S., and Wolska, K. I. (2012). Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine, 19(6), 515-519.
Obire, O. M. O. K. A. R. O., and Ogbonna, S. I. (2017). Antimicrobial activity of some seed extracts on bacteria isolated from Maize slurry (Akamu) in Port Harcourt Metropolis. Science and Technology, 4(1), 188-202.
Okigbo, R. N., and Omadamiro, O. D. (2004). Antimicrobial effect of leaf extract of pigeon pea(Cajanus cajan L.) on some human pathogens. Journal of Herbs, Spices and MedicinalPlants. 12:104-109.
Okigbo, R.N., Anuagasi, C.L., Amadi, J.E. and Ukpabi, U.J. (2009). Potential inhibitoryeffects of some African tuberous plant extracts on Escherichia coli, Staphylococcusaureus and Candida albicans. International Journal of Integrative Biology. 6(2):91-98.
Suaifan, G. A. R. Y., Shehadeh, M., Darwish, D. A., Al-Ijel, H., Yousef, A. M., and Darwish, R. M. (2012). A cross-sectional study on knowledge, attitude and behaviour related to antibiotic use and resistance among medical and non-medical university students in Jordan. Afr J Pharm Pharmacol, 6(10), 763-770.
Waihenya R. K., Mtambo M.M.A and Nkwengulila G., (2002). Evaluation of the efficacy of the crude extract of Aloe secundiflora in chickens experimentally infected with Newcastle Disease Virus. J. Ethnopharmacol. 79:299-304.
About this Article
Cite this Article
APA
Akele, O. E., Osazuwa C. O., Kelly B. A., Adejuigbe, A. & Oyewale, M. B. R.(2025). Antimicrobial Activity of Eleusine indica and Carica papaya Root against Multi-Drug Resistant Clinical Isolates. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Akele, O. E., Osazuwa C. O., Kelly B. A., Adejuigbe, A. and Oyewale, M. B. R. 2025. “Antimicrobial Activity of Eleusine indica and Carica papaya Root against Multi-Drug Resistant Clinical Isolates.” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
5 October 2024
Accepted
10 January 2025
Published
4 February 2025
Corresponding Author Email: emmkel2005@yahoo.com
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














