Okoronkwo, N. C.1 Mbaeyi-Nwaoha, I. E. 1 & Onyeaka, H.2
1Department of Food Science and Technology, University of Nigeria, Nsukka, Nigeria
2Department of Chemical Engineering, University of Birmingham, England, United Kingdom
*Corresponding Author Email: ngozi.okoronkwo@unn.edu.ng…
Highlights
- Four yeast isolates tolerated up to 23% ethanol.
- Two isolates produced 14.3% and 13.2% ethanol from sugarcane molasses.
- Optimal production occurred at pH 4.39, 35°C, and 30° Brix.
- Isolates identified as Saccharomyces cerevisiae and Kluyveromyces marxianus.
- Results support use of indigenous yeasts for industrial bioethanol production.
Abstract
One essential B vitamin that is needed in numerous metabolic pathways is folate. Isolation and characterisation of lactic acid bacteria (LAB)from traditional fermented legumes and cereals was conducted in order to identify suitable strains capable of producing high folate as starter cultures. By adding methotrexate, a folate analogue, to the isolation medium, the folate-producing microbes were separated. The isolated bacteria underwent morphological, biochemical, and phenotypic characterization. They were also evaluated for folate production in a culture medium free of folate (Folic Acid Casei Medium (FACM)), and during milk fermentation. While the 3-aminophenol spectrophotometric approach was used to quantify the synthesis of folate, phenotypic characterisation was carried out using API 50 CHL. The isolates were compared with microbial strains procured from gene bank (USDA); Lactobacillus delbruckii sub. bulgaricus (B59387) and Lactobacillus plantarum (B1848). From the results obtained, 7 isolates that were capable of synthesising folate from the fermented raw materials were obtained and based on phenotypic characterization; they were identified as L. plantarum, L. brevis, L. acidophilus, Lactococcus and Streptococcus species. Four strains out of the nine microbial strains grew in FACM. Total folate content varied between 1.90 to 86.2 µg/ml among the strains. The strains significantly (p<0.05) increased the folate concentration in fermented milk by triple of their initial values. Microorganisms isolated from fermented pigeon pea and rice produced low folate amount in comparison to the microbial strains procured from the gene bank. This study shows that microorganism isolated from fermented brown rice and pigeon pea can synthesize folate in growth media as well as fermented milk. Utilizing them can help prevent folate insufficiency through preventative methods or intervention research. Food manufacturers could leverage the study’s findings to create fermented foods with enhanced folate content, providing consumers with a nutritious and healthy option.
Keywords: Folate, Lactic acid bacteria, Nutritional deficiency, Fermentation.
1. Introduction
A water-soluble vitamin that is vital to the B group family, folate (also known as folic acid or vitamin B9) has a recommended daily intake of 200–400 µg. It is essential for the synthesis of methylation, nucleic acids, and certain proteins and amino acids needed for human growth and replication (Jacob, 2000; Lucock, 2000). According to Rahman et al. (2015), folate plays a major part in the process of protein production, ribonucleic acid (DNA) and deoxyribonucleic acid (RNA) digestion, and the growth of new cells. Folate is also necessary for appropriate fetal growth and development in order to reduce the risk of neural tube abnormalities, which are birth disorders affecting the brain and/or spinal cord. To avoid nutritional deficiency, folate must be obtained externally because humans are unable to manufacture it (Kariluoto et al., 2014). Folate deficiency is a public health concern that significantly impacts expectant mothers and inexorably affects the fetus’s development. By mandating the folic acid fortification of cereal products, a number of nations have worked to guarantee adequate folate intake and prevent the illnesses associated with folate insufficiency (Food fortification initiative, 2015). In most countries, food fortification programs are not always efficient since there aren’t enough control systems, according to Youngblood et al. (2013). However, because of its possible negative consequences, folic acid has not been adopted by many nations as part of a National Fortification Program (Laino et al., 2013). Among other things, excessive folic acid absorption—the chemically synthesized version of folate—may conceal the signs of vitamin B12 insufficiency, which could lead to irreversible neuropathy development (FAO/WHO, 2005). Populations in danger, such as youngsters and pregnant mothers, may not be able to obtain enough folate through tablets and their regular meals due to the aforementioned circumstances. Given the risks associated with folic acid enrichment, an increasing number of researchers are looking into enriching foods with naturally occurring forms of folate instead (Iyer et al., 2009; Leblanc et al., 2007). Conversely, natural forms of folate, including those present in food or synthesized by specific microbes, do not negatively impact an individual’s health, even in cases where excessive consumption occurs (Laino et al., 2013). According to numerous researches, certain strains of lactic acid bacteria (LAB) used as starting cultures in fermentation process have the ability to produce, release, and/or enhance B-group vitamins (Levit et al., 2021; Ngene et al., 2019; Laino et al., 2013; Laino et al., 2012; Padalino et al., 2012; Iyer et al., 2010; Kariluoto et al., 2014; Crittenden et al., 2003; Lin & Young, 2000). In order to produce meals fortified with natural folate through fermentation, the use of lactic acid bacteria strains that produce folate so appears as an alternative (Laino et al., 2013).
2. Materials and Methods
2.1 Procurement of Raw Material
Brown rice was purchased from the National Cereal Research Institute in Badeggi Bida, Niger State, and red pigeon pea (Cajanus cajan L.) was obtained from Ogige market in Nsukka LGA of Enugu state, Nigeria. After being cleaned, all the materials were placed in jute bags and kept at room temperature (30±2℃) until needed. The Agricultural Research Service (ARS) Culture Collection Center, USA, and the United States Department of Agriculture (USDA) provided L. delbruckii sub. bulgaricus (B59387) and L. plantarum (B1848).
2.2 Preparation of Samples
2.2.1 Microorganisms and Growth Conditions
Along with the isolated microorganisms from fermented rice and pigeon pea, the lactic acid bacteria obtained from USDA, Lactobacillus delbruckii sub. bulgaricus (B59387) and Lactobacillus plantarum, (B1848) were preserved at -20°C in a blend of De Man-Rogosa-Sharpe (MRS) broth (De Man Rogosa Sharpe, 1960) and glycerol (20%). The isolates were revived in MRSB and incubated at 37°C for 24 hours prior to use.
2.3 Fermentation of Pigeon Pea and Brown Rice
To enable fermentation, the brown rice and red pigeon pea samples were washed and immersed in distilled water in two separate containers for three days at room temperature. The fermented samples were brought to the laboratory for additional examination.
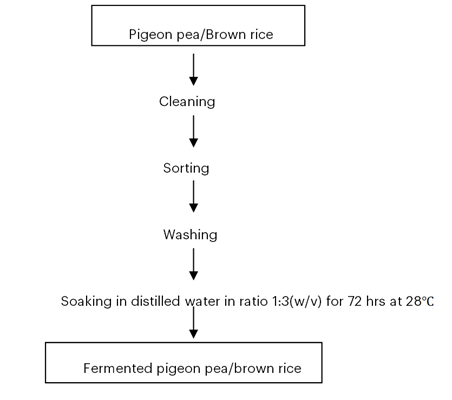
Figure 1: Flowchart for the fermentation of pigeon pea and brown rice
2.4 Isolation of Folate-Producing Microorganisms
Using the pour plate approach as outlined by Harrigan and McCance (1976), bacteria were isolated from the fermenting raw materials. Test tubes were cleaned, and then nine milliliters (9 ml) of distilled water were added. The test tubes were autoclaved for 15 minutes at 121˚C and left to cool. To isolate lactic acid bacteria, deMan Rogosa and Sharpe (MRS) agar were used while malt extract agar was used for yeasts. One milliliter of the fermenting materials were diluted serially using distilled water, and thereafter it was pour plated. After cooling to around 40˚C, the media was aseptically supplemented with 50 mg/ml of methotrexate, an analogue of folic acid. This is to filter the samples for bacteria that produce folate. For 48 hours, lactic acid bacteria and yeasts were incubated at 30± 5˚C. Following incubation, the formation of different colonies was monitored on the plates. To acquire pure culture, these colonies were counted, randomly picked, and streaked.
2.5 Phenotypic Characterization of the Lab and Yeast Isolates
The examination of morphological and biochemical characteristics as reported by Harrigan and McCance (1976) and Kurtzman et al. (2011), respectively, was used to characterize the LAB and yeast isolates. Using the Genera of Lactic Acid Bacteria (Wood and Holzapfel, 1995), the presumed identification of lactic acid bacteria isolates was carried out.
2.5.1 Biochemical characterization
The production of gas, the capacity to grow at various temperatures, pH, and salt tolerance, as well as other fundamental biochemical characteristics was measured. After a 48-hour incubation period, gas generation was monitored by utilizing inverted Durham’s tubes to look at the gas that formed from glucose in MRS broth. Visual evaluation was conducted after 24, 48, and 72 hours of incubation to assess the isolated bacteria’s growth capacity at various temperatures (15, 37, and 45°C). In addition, growth was assessed in MRS agar at pH 4.5, 6.5, and 9.6. The pH was altered using HCl and NaOH, respectively. MRS broth with 2, 3, and 4% (w/v) sodium chloride (NaCl) was used to test salt tolerance, and the cultures were incubated for 72 hours at 37 degrees Celsius.
2.6 Folate Production Screening and Quantification in Culture Medium
The Ruengsitagoon and Hattanat (2012) method was used to screen the acquired isolates for folate synthesis. To put it succinctly, the isolates were cultured in their corresponding broths and incubated for 48 hours. The broth was then centrifuged for 20 minutes at 3000 g. When one milliliter of the supernatant was combined with one milliliter of 4 mol∙L−1 hydrochloric acid, one milliliter of sodium nitrate (1% w/v), one milliliter of sulfamic acid (1% w/v), and one milliliter of 3-amino phenol (1% w/v), an orange-yellow complex was produced, signifying the presence of folate. Using a UV visible spectrophotometer (Jenway, Essex, UK), the complexation’s absorbance was measured at 460 nm. Using a standard curve created with the absorbance of folic acid at a given concentration, the amount of folate present in the samples was calculated. By introducing the isolates into Folic Acid Casei Medium, the isolates’ capacity to produce folate was further assessed. As per Kodi et al. (2015), Folic Acid Casei Medium (FACM) is an assay medium that includes all the necessary nutrients and substances needed for the test organism’s growth, with the exception of folic acid. Potential manufacturers of folate were the isolates that flourished in this chemically defined medium.
2.7 Folate production in fermented milk
Two percent (2%) (w/v) of selected folate-producing bacteria were added into reconstituted non-fat powdered milk, and the mixture was cultured for 24 hours at 37 and 42 °C. Samples were collected at various intervals (0, 6, 8 and 24 hours after inoculation) in order to assess folate synthesis and bacterial growth. A mixture of 500 µL protection buffer and 500 µL milk samples was used to determine the amount of folate. The resultant slurry was centrifuged at 10,000 g for 6 minutes after being heated at 100 °C for 5 minutes to precipitate proteins and liberate folate from milk-binding proteins. Before being utilized for the measurement of total folate, the supernatant was collected and kept at -70°C.
3. Results & Discussion
3.1 Isolation and biochemical characterization of folate producing microorganisms from the natural fermentation of pigeon pea and brown rice
The result for the isolation and biochemical characterization of starter culture from the natural fermentation is presented in Table 1 and Figure 2.
Pigeon pea and brown rice fermentation slurry produced microorganisms that synthesized folate. The development of microbes from the slurry in culture media supplemented with methotrexate (50 mg/ml), a folate analogue, indicates that these isolates are immune to the drug, this was discovered to be the reason of the isolates’ ability to produce folate. This could be explained by the fact that these microbes used the folate analogue to produce necessary components including DNA, RNA, and mRNA, which allowed for their growth and replication (Okoroafor et al., 2019). To create a pure culture, the acquired isolates underwent further screening. Seven isolates in all were found from both brown rice and pigeon pea, according to the data.
3.2 Biochemical Characteristics
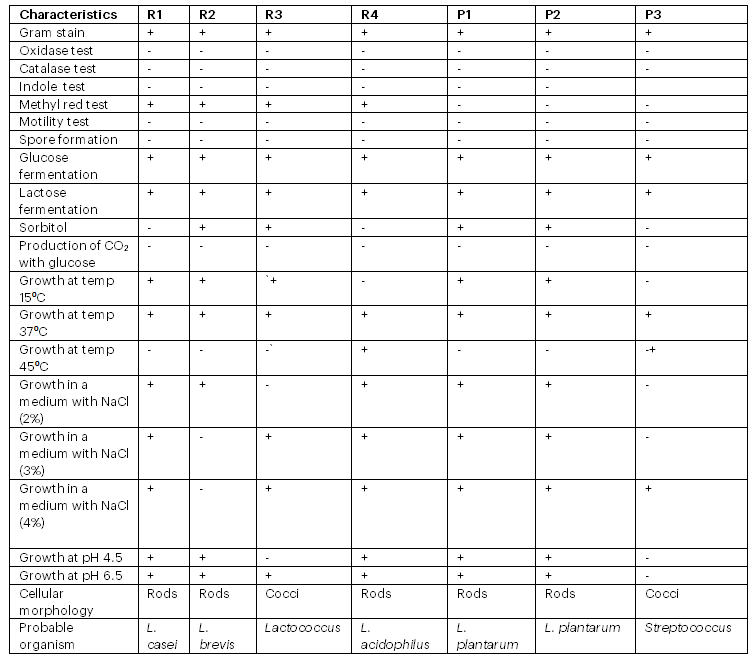
Based on cell shape, gas production from glucose, growth behavior at various temperatures, pH, and salt solutions, the isolates were classified to genus levels. Using the API 50 CHL database, the isolates were further classified to specie levels based on fermentation of carbohydrate. The morphological, physiological, and biochemical examinations revealed a variety of lactic acid bacteria (LAB), categorized according to the genera Streptococcus, Lactobacillus, and Lactococcus. The isolates had a rod- and cocci-shaped appearance, were non-spore-forming, non-motile, gram-positive, and showed negative catalase and oxidase reactions (Table 1). Past research has revealed that Lactobacillus species from fermented legumes and cereals are catalase negative and gram positive (Rao et al., 2015; Salvetti et al., 2012).
Since pH plays a major role in bacterial development, sustainability research in various pH values was conducted. Both acidic and alkaline environments were investigated for each strain. According to the results, the bacteria were able to survive in both extremely acidic and alkaline environments (Table 1). Lactobacillus isolated from fresh fruits and vegetables survived in pH ranges of 3.5 to 7.0, according to a prior work by Pundir et al. (2013). According to Mannan et al. (2017), pH tolerance is an essential criterion for LAB to proliferate and carry out their advantageous function in the digestive system. To determine how well Lactobacillus species tolerated sodium chloride (NaCl), a test was carried out. According to Hoque et al. (2010), NaCl is a hindering chemical that prevents some bacteria from growing. A NaCl tolerance test was performed at doses of 2%, 3%, and 4%. Growth at these concentrations was possible for each isolate.
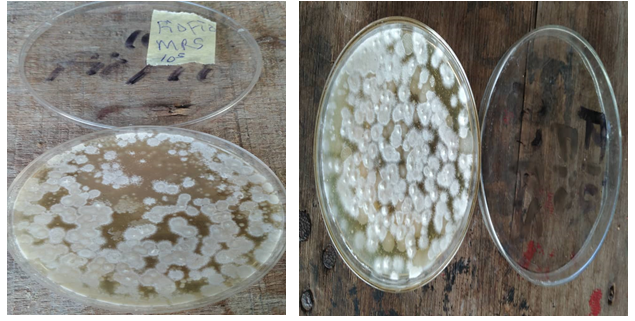
Figure 2: Morphological characteristics of folate-producing microorganisms from pigeon pea
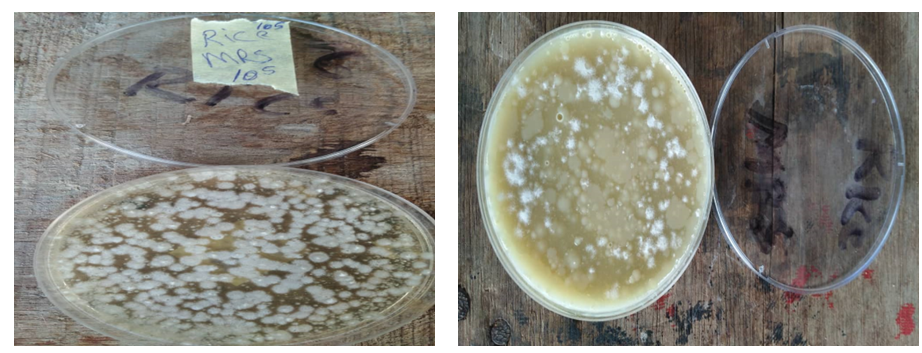
Figure 3: Morphological characteristics of folate-producing microorganisms from brown rice

Figure 4: Pure culture of bacteria isolates from fermented brown rice after streaking
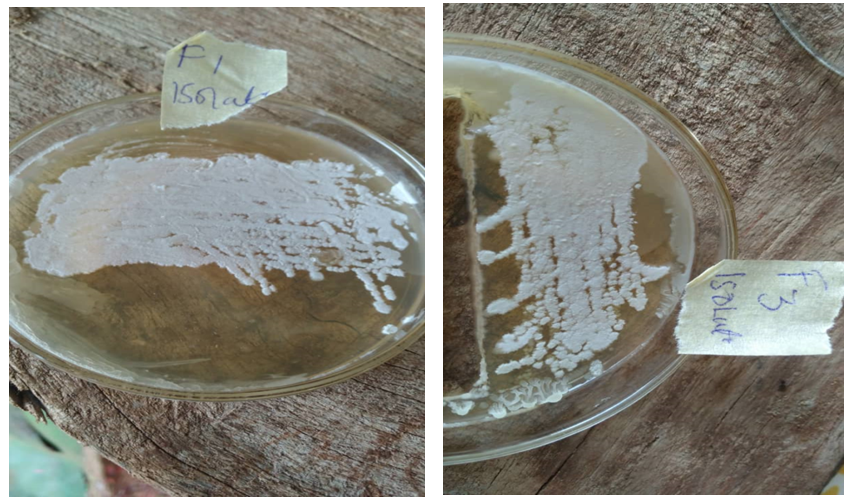
Figure 5: Pure culture of bacteria isolates from fermented pigeon pea after streaking
Table 1: Physiological and biochemical characteristics of isolated strains
3.3 Growth of Lactobacillus Strains in Folate Free Culture Medium (FACM)
Table 2 and Figure 4 and 5 show the growth of lactobacillus strain in FACM.
Natural folate can be synthesized by certain strains of LAB, according to research. Former researchers have reported that some LAB, including L. acidophilus, L. plantarum, L. reuteri, Leuconostoc lactis, Propioni bacterium, and Bifidobacterium longum, have the capacity to generate this vitamin from the B group in addition to some industrially important species, such as Lactococcus lactis and S. thermophiles (Crittenden et al., 2003; Gangadharan et al., 2010; LeBlanc et al., 2011; Lin & Young, 2000; Pompei et al., 2007).
But it’s important to clarify that while some species can manufacture folate; this is a strain-dependent characteristic, meaning that finding strains that produce sufficient levels of this important vitamin requires careful strain selection.
Only four of the seven strains obtained from fermented pigeon pea and brown rice were able to grow after seven subcultures in the folate-free culture medium. It was shown that strains R3 (Lactococcus), R4 (Lactobacillus acidophilus), P1 (Lactobacillus plantarum), P2 (Lactobacillus plantarum), P3 (Streptococcus), B59387 (Lactobacillus delbrucki sub. bulgaricus), and B1848 (Lactobacillus plantarum) were capable of increasing the concentrations of folate in the culture media, but that effect was strain-dependent; that is, certain strains produced significant concentrations of the vitamin, while others produced hardly perceptible levels (Table 2 and Figure 4 and 5). No discernible growth was observed in the culture media for strains R1 (Lactobacillus casei) and R2 (Lactobacillus brevis). Though some strains of bacteria may not require folate for growth, Laino et al. (2012) stated that, growth in the absence of folate does not indicate that these microorganisms manufacture it. Nevertheless, it is a useful indicator and a useful tool for identifying possible LAB that synthesise folate. The range of total folate levels in the strains was 1.90 to 86.2 µg/ml. Isolate R3 (Lactococcus) produced the least amount of folate among the lactic acid bacteria, producing 1.90 µg/ml, while strain B59387 (Lactobacillus delbrucki sub. bulgaricus) produced the most, at 86. 2 µg/ml (Table 2). The microbial strains obtained from the gene bank produced more folate than the bacteria acquired from the fermented raw materials. Despite the fact that it is usually challenging to equate data on entire folate content of LAB with current literatures as a result of variations in the procedure and in culture media used in various research, the entire folate concentrations obtained in this study are in the same range as previously reported folate concentrations (10.98 to 30.89 µg/l) produced by LAB isolated from fermented maize. (Okoroafor et al., 2019). From the results obtained, the maximum folate production in MRS medium among the isolated organisms from the fermented raw material was found to be L. plantarum P1. L. fermentum and L. plantarum, produced a folate concentration of 110 µg/ml and 120 µg/ml respectively in MRS medium in Burkina Faso’s ben salaaga, an African grain gruel. (Greppi et al., 2017). In a different investigation, Lactobacillus plantarum and Lactococcus sp. isolated from Malaysian tapai ubi generated, in MRS media, 8.608µg/L and 10.71µg/L, respectively (Nor et al., 2008). Furthermore, strains of 36 LAB grown in a medium that is free of folate produced extremely high amounts of extracellular folate, whilst other strains produced very little and were almost negligible (Laino et al., 2012). According to various research (Laino et al., 2012; Greppi et al., 2017), strain dependence may be the cause of the variations in folate concentrations generated by bacteria in culture medium. Furthermore, the differences could be attributed to the techniques used in these investigations, since spectrophotometric and high-performance liquid chromatography (HPLC) methods typically yield more precise quantification of folate concentrations than do microbiological assay and ultra HPLC methods. This is because high-performance liquid chromatography and ultra-high-liquid chromatography aim for the particular folate generated rather than the raw vitamins detected using the spectrophotometric and microbiological assay methods (Kariluoto et al., 2004; Kariluoto et al., 2014). This is caused by differences in the sensitivity of the procedures.
Table 2: Growth of Lactobacillus strains in folate- free culture medium and folate production
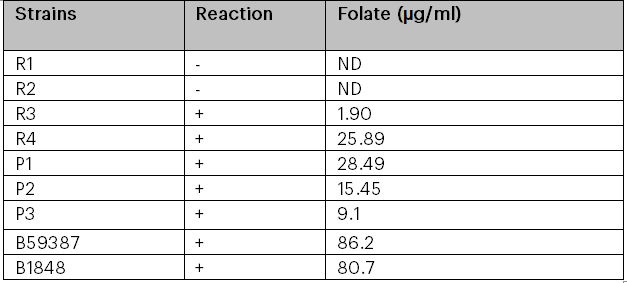
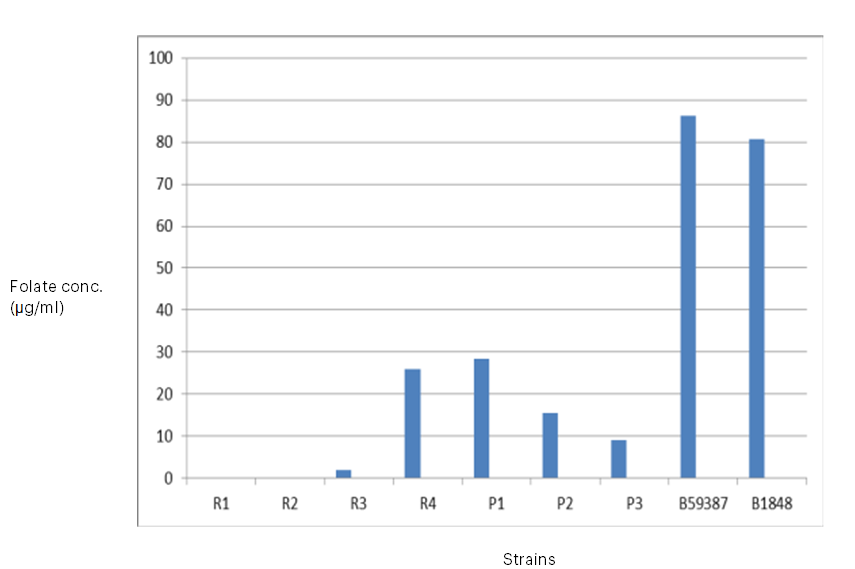
CODES: R1-R4: folate-producing bacteria isolates from brown rice; P1-P3: folate-producing bacteria isolates from pigeon pea ; B59387: L. delbruki sub. bulgaricus; B1848: L. plantarum Fig 5: Growth in folate-free culture medium (FACM) for 16 h @ 37⁰ C
3.4 Production of Folate in Fermented Milk
Strain production of folate in fermented milk is shown in Figure 6 and 7. Although milk is thought to be a nutritious food, it is not a recommended dietary source of folate (Laino et al., 2012). Milk only has 20–30 µg/L of folate, according to a research study by LeBlanc et al. (2007). However, a lot of dairy products, like yoghurt, are made by microbial fermentations, which may result in the production of folate. From the results obtained, strains B59387, B1848, R4, and P1 were chosen based on their increased vitamin synthesis in FACM. The synthesis of folate of the four chosen lactic acid bacteria strains was assessed in fermented milk at various intervals after development in an incubator at 37 and 42℃. The findings revealed that, at 37℃, L. plantarum (B1848) increased the amount of folate significantly (p<0.05) to approximately 30 percent following 24 hours of growth in an incubator whereas L. delbrueckii subsp. bulgaricus (B59387) increased the quantity of folate to approximately 25 percent following 24 hours of fermentation, in comparison with the beginning values. This indicates that the lactic acid bacteria were capable of synthesizing folate rather than consuming the vitamin. The results showed that the maximum folate concentration was achieved after 6 hours of fermentation, followed by 24 hours. L. delbrueckii subsp. bulgaricus (B59387) strain exhibited significantly, dissimilar behavior at 42℃ incubation temperature compared to 37℃. Following a 6-hour fermentation period, B59387 raised the folate concentration of the milk to approximately 40% and then brought it back to the initial levels after a 24-hour period. This decrease may have occurred because the microorganisms responsible for producing folate began consuming it, as they required it for growth and metabolism (Gangadharan and Nampoothiri, 2011; Kundu and Deep, 2014). The results of Laino et al. (2012), who observed that the production of folate by a folate-producing starter culture peaked after 24 hours of fermentation, are consistent with the results gotten. For each of the strain, the same pattern was noted. Results showed that the presence of folate in milk had no effect on folate production by the strains. This is extremely important since certain strains have the ability to increase the quantity of folate in meals beyond what the bacteria require to grow. Higher amounts of folate in fermented milk have been linked to the production of folate by lactic acid bacteria, including Lactobacillus delbrueckii var. bulgaricus and Lactococcus sp. (Laino et al., 2012). The strains in the culture broth (FACM) produced significantly less folate than those in fermented milk, indicating that the presence of folate precursors or the makeup of the food matrix determine how much folate may be produced (Greppi et al., 2017). According to guidelines from the World Health Organization, adults should ingest 400 µg of folate per day, while children should consume 200 µg (FAO/WHO, 2002). Furthermore, it has been stated that if a meal provides at least 10% to 20% of the daily needed amount of folate, it is deemed to be a rich source of the nutrient (Ohio State University, 2005). In this study, milk products containing more than 80 µg/L of folate were produced by certain strains. As an alternative to using these strains separately, combining them could result in a product that has enough folate to meet the 10% Recommended Dietary Allowance for adults and the 20% Recommended Dietary Allowance for children. According to Okoroafor et al. (2019), the use of mixed starter cultures containing both yeast and lactic acid bacteria that produce folate has been shown to boost the amount of folate in fermented food more than the use of either bacterium or yeast as a single starter.
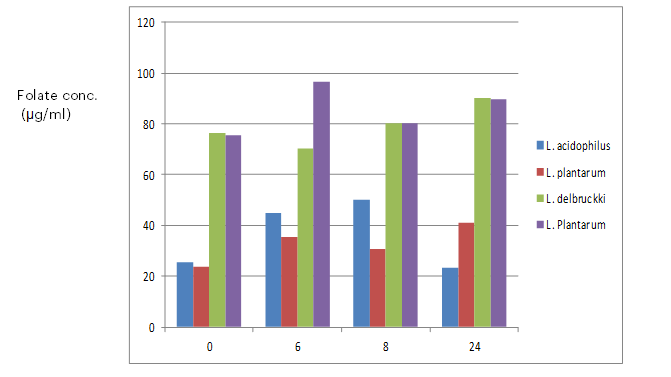
Figure 7: production of folate in fermented milk at 37⁰C. Values are means ± standard deviation (SD) of duplicate determinations
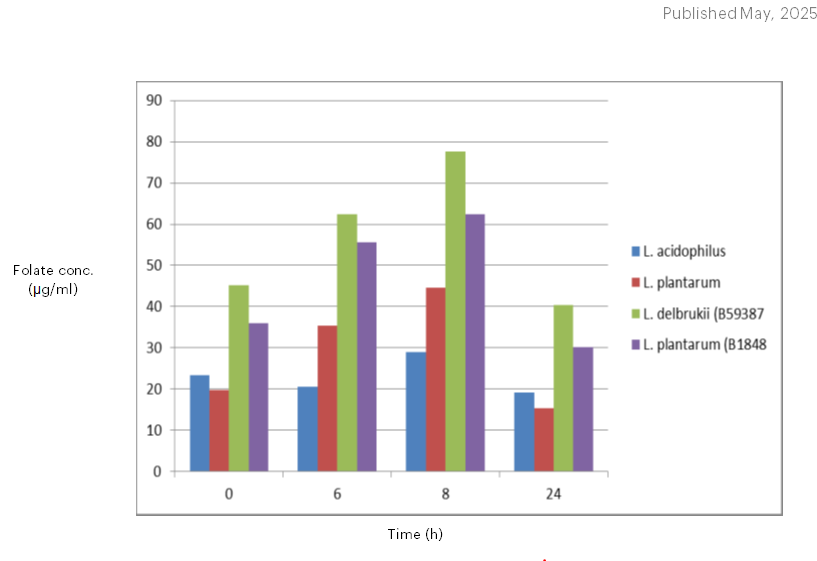
Figure 8: Production of folate in fermented milk at 42⁰C. Values are means ± standard deviation (SD) of duplicate determinations
4. Conclusion
This work demonstrates the ability of microorganisms derived from fermented brown rice and pigeon pea to synthesize folate in both fermented milk and culture media. While the microbial strains obtained from the gene bank showed greater folate values, the strains obtained from the fermented raw materials could potentially have a higher folate concentration if folate precursors were added to the growth media and milk. Fermentation time influenced the folate-producing capacity of the strains. In low-income countries, the use of these strains in combination rather than singly as starter cultures for fermented food production could be a way to contribute to daily folate requirements and boost folate intake. Utilizing them can help prevent folate insufficiency through preventative methods or intervention research. Similarly, the chosen microbes can be bioengineered to enhance their folate-producing capabilities, hence increasing the amount of folate in fermented meals.
Acknowledgement
The authors would like to thank the Agricultural Research Service (ARS) Culture Collection Center, USA, of the United States Department of Agriculture (USDA) for providing the microbial culture utilized in this study. One cannot overstate the impact you have.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
Crittenden, R., Martinez, N., and Playne M. (2003). Synthesis and utilization of folate by yoghurt starter cultures and probiotic bacteria. International Journal of Food Microbiology, 80:217-222.
De Man, J. D., Rogosa, M., and Sharpe, M. E. (1960). A medium for the cultivation of lactobacilli. Journal of Applied Bacteriology, 23, 130- 135.
FAO/ WHO (2005). Vitamin and mineral requirements in human nutrition, Second edition. http://www.who.int/nutrition/publications/micronutrients/9241546123/en/.
FAO/WHO (2002). Human vitamin and mineral requirements. In FAO/WHO (Ed.), Human vitamin and mineral requirements. Bangkok, Thailand.
Food Fortification Initiative (2015). Enhancing Grains for Healthier Lives (http://www.ffinetwork.org/index.html)
Gangadharan D., Sivaramakrishnan S, Pandey A. and Nampoothiri, K. M. (2010). Folate-producing lactic acid bacteria from cow’s milk with probiotic characteristics. International Journal of Dairy Technology, 63(3):339-348. https://doi:10.1111/j.1471-0307.2010.00590.x
Gangadharan, D. and Nampoothiri, K.M. (2011) Folate Production Using Lactococcus lactis sp cremoris with Implications for Fortification of Skim Milk and Fruit Juices. Food Science Technology, 12, 1859-1864. https://doi.org/10.1016/j.lwt.2011.05.002
Greppi, A., Hemery, Y., Berrazaga, I., Almaksour, Z. and Humblot, C. (2017) Ability of Lactobacilli Isolated from Traditional Cereal-Based Fermented Food to Produce Folate in Culture Media under Different Growth Conditions. Food Science and Technology, 86, 277-284. https://doi.org/10.1016/j.lwt.2017.08.007
Harrigan, W.F. and McCance, M.F. (1966) Laboratory Methods in Food and Dairy Microbiology. 2nd Edition, Academic Press, London.
Hoque, M.Z., Akter, F., Hossain, K.M., Rahman, M.S.M., Billah, M.M. and Islam, K.M.D. (2010). Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World Journal of Dairy Food Science, 5, 39-46.
Iyer, R., Tomar, S. K., Singh, R., and Sharma, R. (2009). Estimation of folate in milk by microbiological assay using tri-enzyme extraction method. Milchwissenschaft, 64(2), 125-127.
Iyer, R., Tomar, S., Kapila, S., Mani, J. and Singh, R. (2010). Probiotic properties of folate producing Streptococcus thermophilus strains. Food Resource International, 43:103-110.
Jacob, R. A. (2000). Folate, DNA methylation and gene expression : factors of nature and nurture. American Journal of Clinical Nutrition, 72: 903-904.
Kariluoto, S., Edelmann, M., Nyström, L., Sontag-Strohm, T., Salovaara, H., Kivelä, R. and Piironen, V. (2014) In Situ Enrichment of Folate by Microorganisms in Beta-Glucan Rich Oat and Barley Matrices. International Journal of Food Microbiology, 176: 38-48. https:// doi.org/10.1016/j.ijfoodmicro.2014.01.018
Kariluoto, S., Vahteristo, L., Salovaara, H., Katina, K., Liukkonen, K. and Piironen V. (2004) Effect of Baking Method and Fermentation on Folate Content of Rye and Wheat Breads. Cereal Chemistry, 81, 134-139. https://doi.org/10.1094/CCHEM.2004.81.1.134
Kodi, C., Gothandam, K.M. and Prabakaran, G. (2015). Identification and Characterization of Folic Acid Producing Potential Starter for Curd Fermentation. International Journal of Current Microbiology and Applied Sciences, 4, 118-130.
Kundu, S. and Deep, S. (2014) Comparative Studies on Folate Production and Parameter Optimization in Fermented Milk from Yoghurt Starter Culture. International Journal of Engineering Sciences and Research Technology, 3, 653-660
Kurtzman, C.P., Fell, J.W. and Boekhout, T. (2011). The Yeasts: A Taxonomic Study. 5th Edition, 3 Vol., Elsevier Science Publishers, Amsterdam.
Laiño, J.E., Juarez del Valle, M., Savoy de Giori, G. and LeBlanc, J.G. (2013). Development of a high folate concentration yogurt naturally bio-enriched using lactic acid bacteria. LWT Food Science and Technology, 54: 1-5.
Laiño, J.E., LeBlanc, J.G. and Savoy de Giori, G. (2012) Production of Natural Folates by Lactic Acid Bacteria Starter Cultures Isolated from Artisanal Argentinean Yogurts. Canadian Journal of Microbiology ,58: 581-588.
LeBlanc J. G., de Giori, G.S., Smid, E. J., Hugenholtz, J. and Sesma, F. (2007). Folate production by lactic acid bacteria and other food-grade microorganisms. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. 1:329-339.
LeBlanc, J.G., Laino, J.E., Juarez del Valle, M., Vannini, V., van Sinderen, D., Taranto, M.P., Font de Valdez, G., Savoy de Giori, G. and Sesma, F. (2011). B-Group vitamin production by lactic acid bacteria – current knowledge and potential applications. Journal of Applied Microbiology, 111, 1297–1309. http://refhub.elsevier.com/S0168-1605(17)30509-3/rf0115
Levit, R., Savoy de Giori, G., de Moreno de LeBlanc, A. and LeBlanc, J. G. (2021). Recent update on lactic acid bacteria producing riboflavin and folates: application for food fortification and treatment of intestinal inflammation. Journal of Applied Microbiology, 130(5): 1412-1424. http://doi:10.1111/jam.14854
Lin, M.Y. and Young, C.M. (2000). Folate levels in cultures of lactic acid bacteria. International Dairy Journal, 10:409-413
Lucock, M. (2000). Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Molecular Genetics and Metabolism, 71: 121-138.
Mannan, S. J., Rezwan, R., Rahman, S. and Begum, K. (2017). Isolation and Biochemical Characterization of Lactobacillus species from Yogurt and Cheese samples in Dhaka Metropolitan Area. Bangladesh Pharmaceutical Journal, 20(1): 27-33.
Ngene, A., Onwuakor, C., Aguiyi, J., Ifeanyi, V., Ohaegbu, C., Okwuchukwu, C., Kim, E. and Egbere, J. (2019). Screening of some lactic acid bacteria isolated from selected Nigerian fermented foods for vitamin production. Advances in Microbiology, 9, 943-955. http://doi:10.4236/aim.2019.911060
Nor, N. M., Mousavi, S. S, Mohamad, R., Ling, F. H. and Rahim, R. A. (2008). Screening of Lactic Acid Bacteria for Folic Acid Production from Various Sources. In: 30th Symposium of Malaysian Society for Microbiology; Malaysia.
Ohio State University. (2005). Extension fact sheet of Folate (folacin, folic acid). In Ohio State University extension fact sheet of Folate (folacin, folic acid), HYG 5553- 05 (pp. 1-3)
Okoroafor, I., Banwo, K., Olanbiwoninu, A. A. and Odunfa, S, A. (2019). Folate Enrichment of Ogi (a Fermented Cereal Gruel) Using Folate Producing Starter Cultures. Advances in Microbiology, 9, 177-193. https://doi.org/10.4236/aim.2019.93014
Padalino, M., Perez-Conesa, D., Lopez-Nicolas, R., Frontela-Saseta, C. and Ros-Berruezo, G. (2012). Effect of fructooligosaccharides and galactooligosaccharides on the folate production of some folate-producing bacteria in media cultures or milk. International Dairy Journal, 27:27-33.
Pompei, A., Cordisco, L., Amaretti, A., Zanoni, S., Matteuzzi, D. and Rossi, M. (2007). Folate production by bifidobacteria as a potential probiotic property. Applied Environmental Microbiology, 73(1):179-185. https://doi:10.1128/aem.01763-06
Pundir, R.K., Rana, R., Kashyap, N. and Kaur, A. (2013). Probiotic potential of lactic acid bacteria isolated from food samples: an in vitro study. Journal of Applied Pharmaceutical Science, 3, 85-93.
Rahman, T., Chowdhury, M. M. H., Islam, M. T., Akhtaruzzaman, M., (2015). Microbiological assay of folic acid content in some selected Bangladeshi food stuffs. International Journal of Biotechnology, 7, 35.
Rao, K.P., Chennappa, G., Suraj, U., Nagaraja, H., Raj, A.P.C. and Sreenivasa, M.Y. (2015). Probiotic potential of Lactobacillus strains isolated from Sorghum-based traditional fermented food. Probiotics and Antimicrobial. Proteins, 7, 146-156.
Ruengsitagoon, W. and Hattanat, N. (2012) Simple Spectrophotometric Method for Determination of Folic Acid. 4th Annual Northeast Pharmacy Research Conference of Pharmacy Profession in Harmony, 11-12 February 2012, 281.
Salvetti, E., Torriani, S. and Felis, G.E. (2012). The genus Lactobacillus: a taxonomic update. Probiotics & Antimicrobial. Proteins, 4, 217–226. https://doi.org/10.1007/s12602-012-9117-8
Wood, B.J.B. and Holzapfel, W.H. (1995). The Genera of Lactic Acid Bacteria. Vol. 2, Blackie Academic and Professional Glasgow. https://doi.org/10.1007/978-1-4615-5817-0
Youngblood, M.E., Williamson, R., Bell, K.N., Johnson, Q., Kancherla, V., and Oakley, G.P. (2013). Update on global prevention of folic acid- preventable spina bifida and anecephaly. Birth Defects Resources, 97: 658-663.
About this Article
Cite this Article
APA
Okoronkwo N. C., Mbaeyi-Nwaoha I.E. & Onyeaka, H. (2025). Isolation, Biochemical Characterization and Screening of Folate Producing Potential Starter Culture from Pigeon Pea and Brown Rice. SustainE. 1(2). doi:10.55366/suse.v1i2.10. 1-13.
Chicago
Okoronkwo N. C., Mbaeyi-Nwaoha I.E. & Onyeaka, H (2025). Isolation, Biochemical Characterization and Screening of Folate Producing Potential Starter Culture from Pigeon Pea and Brown Rice. SustainE. 1, no. 2 (May 20, 2025). 1-13. https://doi.org/10.55366/suse.v1i2.10
Received
15 October 2024
Accepted
23 March 2025
Published
20 May 2025
Corresponding Author Email: ngozi.okoronkwo@unn.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














