Adegunloye, D. V.;1 Ekundayo, F. O.;1 Olalemi, A. O.;1 Obagade, T. A.;2 Ogundolie, F. A.;3 Ugwuja, A. N.;4 Olusegun-Awosika, B. D.;1 &Ojo, O. J.2
1Department of Microbiology, Federal University of Technology, Akure, PMB 704, Ondo State, Nigeria
2Department of Physics, Federal University of Technology, Akure, PMB 704, Ondo State, Nigeria
3Department of Biotechnology, Baze University, Abuja, Nigeria
4Department of Biology, Adeyemi Federal University of Education, Ondo, Nigeria
*Corresponding Author Email: dvadegunloye@futa.edu.ng …
Abstract
Petrochemical wastes represent a significant environmental hazard due to their toxic and persistent nature, posing severe risks to ecosystems and human health. Microbial fuel cells (MFCs) offer an eco-friendly solution for bioelectricity generation by utilising microbial metabolic processes. This study evaluates the potential of Bacillus pumilus for bioelectricity production using polluted soil and water samples from Agbabu, Nigeria—a region impacted by deposits of petrochemical wastes. Bacteria were isolated from the samples and characterised through cultural and molecular techniques, while gas chromatography–mass spectrometry (GC–MS) was employed to detect petrochemical pollutants. The physicochemical properties of the soil and water were analysed using standard methods. The microbial load of the samples was determined to be 2.3 × 10^5 CFU/g in soil and 1.1 × 10^5 CFU/ml in water, with B. pumilus identified as the dominant organism. MFCs constructed and inoculated with B. pumilus achieved peak performance on the sixth day with water samples, generating a voltage of 1.375 V and a current of 0.242 A, reflecting efficient microbial activity and electron transfer. A voltage of 0.820 V and a current of 0.080 A were generated on the same day for the soil sample. These findings demonstrate the potential of B. pumilus as a sustainable bioelectricity producer and underscore its relevance in renewable energy applications, particularly in regions impacted by petrochemical pollution.
Keywords: Bioelectricity, microbial fuel cells, petrochemical wastes.
1. Introduction
Petrochemical industries significantly contribute to environmental pollution due to the release of toxic waste products, including hydrocarbons and heavy metals (Khaki et al., 2023). These pollutants are persistent in the environment, posing substantial risks to ecosystems and human health. Traditional waste management methods, such as incineration and landfilling, often fall short in addressing the complex and toxic nature of petrochemical wastes, leading to secondary pollution (Johnson & Smith, 2024).
Agbabu, located in the Okitipupa Local Government Area of Ondo State in Nigeria and known for its extensive bitumen deposits, serves as a pertinent case study for assessing the environmental impact of petrochemical activities. The extraction and processing of bitumen in this area have introduced significant pollutants into the environment, particularly affecting soil and water sources. Bitumen, a dense, highly viscous form of petroleum, contains various complex hydrocarbons that are resistant to natural degradation (Oseni et al. 2024). The processing of bitumen in Agbabu has led to the contamination of local ecosystems, posing health risks to the community. Among the primary pollutants are polycyclic aromatic hydrocarbons (PAHs), heavy metals, and volatile organic compounds (VOCs), which have particularly detrimental effects on water quality. Microbial fuel cells (MFCs) offer an innovative approach by combining waste treatment with energy production (Gupta, 2024). MFCs employ bacteria to convert organic matter into electrical energy through bio-electrochemical processes, providing the dual benefit of waste degradation and bioelectricity generation (Logan, 2008). However, there is limited research on the specific application of Bacillus pumilus in MFCs for the degradation of petrochemical wastes, highlighting the need for further investigation (Idris et al., 2022).
2. Material and Methods
2.1 Collection of Polluted Soil and Water Samples
Petrochemical waste samples (soil and water) were collected aseptically from three bitumen-polluted sites in Agbabu village, located within the Odigbo Local Government Area of Ondo State. These samples were collected in sterile specimen bottles, transported to the Microbiology Laboratory at the Federal University of Technology, Akure, and analysed immediately. Surface water samples were collected using sterile glassware at three sampling stations, each located 200 metres apart. Sediment samples were collected 2–3 cm deep using a sterile auger at the same three sampling stations. Control samples were taken from a random well approximately 120 metres away.
2.2 Physicochemical Analysis of Soil, Sediment and Water Samples
The physicochemical parameters of the soil and water samples were analysed. These parameters include turbidity, chemical oxygen demand (COD), biochemical oxygen demand (BOD), percentages of heavy metals, pH, pOH, and hydrocarbon composition.
2.3 Detection of Petrochemical Pollutants in Samples Using GC–MS
The composition of the crude oil used in the study was analysed using gas chromatography–mass spectrometry (GC–MS). Samples were prepared by dissolving 1g of crude oil in 10 ml of dichloromethane. The GC–MS analysis was performed using a Shimadzu QP2010 Ultra system with a DB-5MS column.
2.4 Microbial Fuel Cell (MFC) Construction
Plastic containers (1.5 L) served as the anode and cathode chambers. A hole was bored in the side of each container for fitting a PVC pipe using an electric soldering iron, and another small hole was drilled in the lid for the passage of a copper wire. Wax gum was used to seal the edges of the holes. The setup was tested for leakage by pouring distilled water into the chambers and observing them for one day, after which the water was disposed of according to the method described by Adegunloye and Faloni (2021).
2.5 Preparation of the Salt Bridge
The salt bridge was prepared using 5% sodium chloride (NaCl) and 10% agar–agar. Five grams of NaCl was weighed into an empty pot containing 20 g of agar–agar. One hundred and fifty millilitres of distilled water was added and boiled to dissolve the agar–agar. The agar was allowed to cool to room temperature before being poured into PVC pipes approximately 15 centimetres long and 4 centimetres in diameter. These were covered at one end with nylon and rubber bands. Once the pipes were filled, they were left to stabilise and solidify for about 10 minutes.
2.6 Preparation of Electrodes
Electrodes were collected from dismantled batteries. The battery electrodes were sterilised using 95% ethyl alcohol, and bare copper wires were firmly wrapped around them.
2.7 Coupling of the MFC
The setup was coupled by joining the two chambers together using the salt bridge, which served as the membrane. The anode and cathode chambers were cleaned with 95% ethyl alcohol to eliminate any contaminants. Two hundred and fifty grams of sand was sterilised in 1,200 ml of nutrient broth and 300 ml of bitumen. After cooling, 5 ml of the bacterial inoculum (Bacillus pumilus) was added to the first anodic chamber; this procedure was repeated for the second chamber. In the cathode chamber, potassium permanganate was prepared by dissolving 23.7 g in 1,500 ml of sterilised water, and in the second cathode chamber, 1,500 ml of distilled water was used. Electrodes were carefully dipped into the chambers with the wires passing through the holes on the lids. A multimeter was connected to the anode and copper wires and set to measure direct current and voltage.
3. Results and Discussion
The microbial load of the water and soil samples obtained from Agbabu was determined. The bacterial colony count for the water sample was 2.3 x 105 CFU/ml, while that of the soil sample was 1.1 x 105 CFU/g. These values reflect the bacterial populations observed in both samples.
The bacterial isolate was molecularly identified as Bacillus pumilus. The isolate’s molecular identity has an accession number of EU256393.1 with 99% similarity.

Plate 1: Setup of microbial fuel cell for the production of bioelectricity

Plate 2: Microbial fuel cell setup
The setup of the microbial fuel cell for the production of bioelectricity is represented in Plates 1 and 2. The setup shows the production of the currents and voltage generated.
The physicochemical properties of the soil and water from Agbabu are shown in Table 1. The pH of the soil was slightly acidic (6.73), while the water sample was alkaline (8.3). Total dissolved solids were higher in water (960 mg/L) compared to soil (550 mg/L). The water sample exhibited a turbidity of 13 NTU, and the chloride content was higher in soil (72.89 mg/kg) than in water (10.93 mg/L). Other parameters showed notable differences between the two samples: nitrate (68.69 mg/kg in soil, 13.35 mg/L in water), ammonia (27.45 mg/kg in soil, 16.12 mg/L in water), phosphate (nil in soil, 4.54 mg/L in water), nitrite (2.1 mg/kg in soil, 0.8 mg/L in water), biochemical oxygen demand (BOD) (19 mg/L in soil, 4.65 mg/L in water), chemical oxygen demand (COD) (670 mg/L in soil, 380 mg/L in water), total organic carbon (TOC) (0.12% in soil, 13% in water), and organic matter (2.1 mg/kg in soil, 40 mg/kg in water).
Table 1: Physicochemical Properties of Soil and Water Samples from Agbabu

The chromatograms of the tested samples are represented in Figures 1 and 2. These figures show the retention peaks of both the soil and water samples from Agbabu. Tables 2 and 3 list the detected hydrocarbon compounds, including their retention times, molecular formulas, molecular weights, and percentage abundances. The compounds range from simple hydrocarbons, such as n-nonane, to complex molecules like octyldecanoate. The molecular weights vary between 114 and 691, indicating a mix of light and heavy hydrocarbons. The most abundant compound is 9,12-octadecadienal (29.93%), followed by octyldecanoate (12.40%) and 2,6,11-trimethyldodecane (8.21%). These compounds include aliphatic hydrocarbons, esters, halogenated compounds, and acids, which suggest potential environmental risks. Retention times indicate varying levels of complexity and polarity among the compounds. This data aids in understanding the environmental impact of petrochemical waste and can guide the development of appropriate treatment strategies.

Figure 1: Chromatogram of the Soil Sample

Figure 2: Chromatogram of the Water Sample
Table 2: Identified Hydrocarbon Compounds in the Petrochemical Waste (Soil Sample)

Table 3: Identified Hydrocarbon Compounds in the Petrochemical Waste (Water Sample)
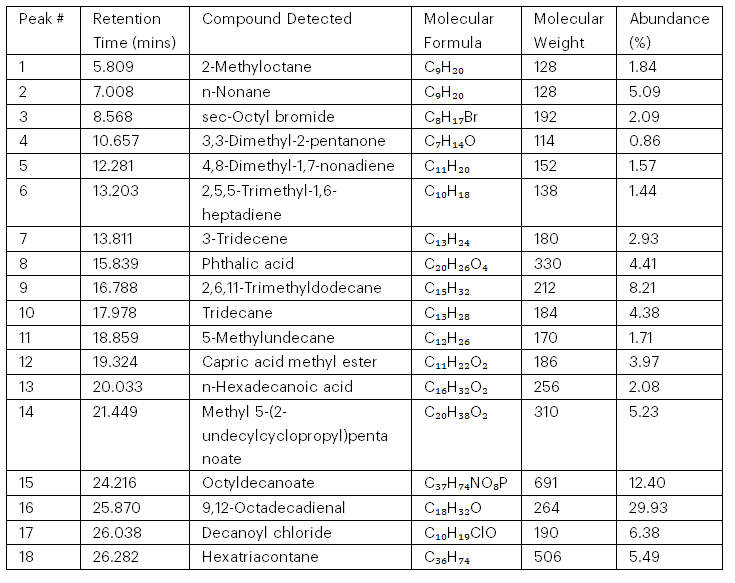
Table 2 identifies 18 hydrocarbon compounds in the petrochemical waste (soil sample) with retention times, molecular weights (114–691), and varying abundances. Notably, 9,12-octadecadienal (29.93%) and octyldecanoate (12.40%) are the most abundant. The compounds include aliphatic hydrocarbons, halogenated compounds, and oxygen-containing molecules, such as phthalic acid and n-hexadecanoic acid, indicating potential environmental risks. The presence of long-chain and complex esters highlights the chemical diversity of the waste. This analysis aids in assessing and managing the environmental impact of petrochemical waste.
Current generated from soil and water from Agbabu with Bacillus pumilus is shown in Figures 3–6. There was a progressive increase in the values of the current and voltage, with the lowest voltage recorded at 0.010 V. This indicates that Bacillus pumilus was able to utilise the nutrients in the medium and is, therefore, highly effective in the generation of bioelectricity. The results demonstrated that the MFC system with Bacillus pumilus generated a stable voltage (approximately 0.5–1.375 V), which is comparable to values reported in previous studies by Zhou et al. (2024) involving Bacillus strains in MFCs. The production of bioelectricity occurred as a result of microbial metabolism, whereby electrons were transferred from the nutrient broth in the anode, through the proton membrane (salt bridge), to the cathode compartment containing KMnO₄, thus creating an electric current (Adegunloye & Faloni, 2021).
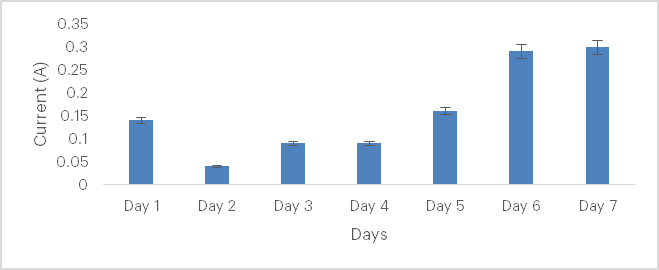
Figure 3: Current generated from soil sample from Agbabu with Bacillus pumilus
Figure 3 shows the variation in current generated over seven days. The current starts high at approximately 0.17 A on Day 1 but drops to 0.05 A on Day 2. From Day 3 onwards, it gradually recovers, reaching 0.18 A by Day 5. Peak current generation occurs on Days 6 and 7 at 0.28 A, indicating a significant increase in activity. This trend highlights an initial decline, followed by consistent recovery and stabilisation at maximum levels.

Figure 4: Voltage generated from soil sample from Agbabu using Bacillus pumilus
The voltage and current both drop initially from Day 1 to Day 2, with the current decreasing more significantly. From Day 3 to Day 5, both show a steady recovery, with the voltage reaching 1.3 V and the current 0.18 A by Day 5. Voltage continues to increase steadily, peaking at 1.7 V on Day 7, while the current peaks earlier at 0.28 A on Day 6 and then stabilises. Overall, voltage shows a more consistent and significant rise compared to current over the seven days. This suggests that the system may be more efficient in maintaining voltage generation relative to current for the soil sample.

Figure 5: Current generated from water sample from Agbabu with Bacillus pumilus

Figure 6: Voltage generated from water sample from Agbabu with Bacillus pumilus
4. Conclusion
The integration of Bacillus pumilus into a microbial fuel cell (MFC) setup resulted in the generation of bioelectricity with stable voltage outputs. This process also facilitated electron transfer, thereby producing bioelectricity through microbial metabolism.
5. Recommendations
Based on the findings of this study, continued research into optimising the efficiency of microbial fuel cells is essential. Future work should focus on improving electrode materials, enhancing biofilm formation, and increasing electron transfer rates to boost power output and scalability. Further studies should also investigate the use of Bacillus pumilus in combination with other microbial species to reduce petrochemical pollutants and maximise energy production.
6. Acknowledgement
The authors are grateful to the TETFund National Research Fund for the award granted to sponsor this work.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Adegunloye, D. V., & Faloni, T. M. (2021). Enhancing electricity generation with the use of KMnO₄ as an electron acceptor in microbial fuel cells. Jordan Journal of Biological Sciences, 14(2), 229–238.
Gupta, A. D. (2024). Microbial fuel cells (MFCs) for waste recycling and energy production. In Biodegradable Waste Processing for Sustainable Developments (p. 220).
Idris, M. O., Kim, H. C., Yaqoob, A. A., & Ibrahim, M. N. M. (2022). Exploring the effectiveness of microbial fuel cells for the degradation of organic pollutants coupled with bio-energy generation. Sustainable Energy Technologies and Assessments, 52, 102183.
Johnson, R., & Smith, P. (2024). Advances in the treatment and disposal of petroleum waste. Journal of Environmental Management, 67(3), 102–115.
Khaki, E., Boleydei, H., Abyar, H., & Nowrouzi, M. (2023). Integrating eco-environmental assessment with energy recovery for petrochemical wastewater treatment technologies: A transition towards green and sustainable management. Journal of Water Process Engineering, 55, 104103.
Logan, B. E. (2008). Microbial fuel cells. John Wiley & Sons.
Oseni, Y. M., Aroke, U., & Jibril, B. (2024). Optimised condition catalytic upgrading of Agbabu bitumen in the presence of rice husks. Path of Science, 10(4), 6001–6021.
Zhou, Y., Zhang, J., & Liu, S. (2024). Energy generation from organic waste using Bacillus pumilus based microbial fuel cells. Renewable Energy, 170, 1068–1080.
About this Article
Cite this Article
APA
Adegunloye, D. V., Ekundayo, F. O., Olalemi, O. A., Obagade, T. A., Ogundolie, F. A., Ugwuja, A. N., Olusegun-Awosika, B. D. & Ojo, O. J. (2025). Assessment of Bacillus pumilus from Petrochemical Wastes from Agbabu and Production of Bioelectricity Using Microbial Fuel Cells. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Adegunloye, D. V., Ekundayo, F. O., Olalemi, O. A., Obagade, T. A., Ogundolie, F. A., Ugwuja, A. N., Olusegun-Awosika, B. D. and Ojo, O. J.2025. “Assessment of Bacillus pumilus from Petrochemical Wastes from Agbabu and Production of Bioelectricity Using Microbial Fuel Cells” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
125 November 2024
Accepted
10 January 2025
Published
4 February 2025
Corresponding Author Email: dvadegunloye@futa.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














