Omoya, F. O. 1 Salami, F. F 2 & Ajayi, K. O. 3
1Department of Microbiology, Federal University of Technology, Akure.
2Department of Medical Laboratory Science, Federal University of Technology, Akure.
3Department of Natural Science (Microbiology), Precious Cornerstone University, Ibadan.
*Corresponding Author Email: olusola-makinde@futa.edu.ng …
Abstract
Microorganisms grow on surfaces to form biofilms to adapt and protect from environmental stresses. This study aimed to determine the occurrence of antimicrobial-resistant biofilm-producing microorganisms in hospital environment and the effectiveness of essential oil from Azadirachta indica leaf as a control of biofilm-producing microorganisms. Swabs of surgical equipment (SE) and high-touch surfaces (HTS) were collected Hospitals in, and microorganisms were isolated, isolates were screened for biofilm production using standard microbiological methods. The biofilm producing isolates were subjected to commercial antibiotics using the disc diffusion method, and isolates resistant to more than three classes of antibiotics were used for further study. Neem leaves were collected, and the essential oil was extracted using the hydro-distillation method. Biofilm-producing bacteria showed higher antibiotic resistance to gentamicin (72.50%), amoxicillin-clavulanic acid (52.50%) and cefixime (35%). Essential oil from Azadirachta indica leaf had higher inhibitory effects against antibiotic-resistant biofilm-producing bacteria (B. niacin, E. aerogenes, P. aeruginosa and S. lentus) and fungi (Aspergillus niger, Aspergillus flavus, Fusaruim culmorum and Aspergillus penicilloides). The chemical compounds such as 1R-.alpha.-Pinene (47.13%) and Ocimene (21.04%) were the most abundant in the essential oil of A. indica leaf. The presence of these bioactive compounds that inhibited biofilm-forming microorganisms in the oil could be that it also serves as an antimicrobial. Hence, it could be used as a disinfectant in the hospital environment to control biofilm-forming microorganisms.
Keywords: Biofilm, bacteria, fungi, hospital, antibiotic-resistant.
1. Introduction
Biofilms are ubiquitous in healthcare settings; by nature, their susceptibility to antimicrobials is lower, and they are implicated in different healthcare-associated infections (HAI). Their antimicrobial resistance ability is due to the presence of a matrix (extracellular DNA) and the composition of the biofilm environment (Maillard & Centeleghe, 2023). In a hospital environment, biofilm occurs in two ways: hydrated biofilms and environmental dry biofilms (DSB). They protect different pathogens from being eliminated by antimicrobials and enhance successful colonization of the hospital environment and medical devices; therefore, there is a need for different approaches to eliminate them (Gupta et al., 2016; Sharma et al., 2023; Maillard & Centeleghe, 2023; Odelola et al., 2023). Examples of bacterial pathogens that are commonly isolated in biofilm are Pseudomonas aeruginosa, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Streptococcus viridans, Staphylococcus aureus, and Enterococcus faecalis (Chen et al., 2013; Odelola et al., 2023). As a result of the presence of these pathogens, 70% of all human microbial infections are due to biofilms and are implicated in diseases like chronic wounds, endocarditis, periodontitis, cystic rhinosinusitis, fibrosis, meningitis, kidney infections, and prosthesis. (Paharik & Horswill, 2016; Vandana, 2021).
Once biofilm is formed in bacteria, the likelihood of gaining antibiotic resistance increases by 10 to 1000-fold compared to bacteria in non-biofilm environment (Yang et al., 2021; Sharma et al., 2023). The occurrence of antibiotic-resistant bacteria like Beta Lactamase-producing Escherichia coli and Methicillin-resistant Staphylococcus aureus in hospitals is well documented (Maillard & Centeleghe, 2023; Sharma et al., 2023). They can resist drugs by using different mechanisms, such as decreasing drug penetration (Liu et al., 2022), modification of chemicals in the microenvironment (Dan et al., 2023), and differentiation to spore formation (Liu et al., 2022). However, when bacteria within the biofilm can survive very high concentrations of antibiotics, it results in a condition known as recalcitrance (Sharma et al., 2019).
It is very difficult to control biofilm once it forms and it may increase the frequency and severity of infections, thereby increasing the mortality and morbidity in infection (Algburi et al., 2017). However, it can be reduced and eliminated with the help of different methods (Brown et al., 2015; Jiang et al., 2020). Several physical, biological, medicinal, surface and hand combinatorial techniques have been applied in clinical settings to dissolve mature biofilms. (Jiang et al., 2020). Various antibacterial agents, dietary supplements, anti-surface attachment treatments, and changes in ambient conditions have been used against biofilm growth.
The neem tree contains hundreds of compounds (i.e., phytochemicals), many of which have more than 300 unique bioactive compounds (e.g., azadirachtin, gedunin, and nimbolide) that have drug potentials (Saleem et al., 2018; Braga et al., 2020; Nagini et al., 2021). The plant can treat biofilm infections by quorum sensing inhibitors, efflux pump inhibitors, and metal chelators, which are all potentially powerful antibiofilm agents (Borges et al., 2016). A. indica extracts has activity against biofilm-forming strains of S. aureus and methicillin-resistant S. aureus (MRSA) (Kaur et al., 2021). Petroleum ether neem extract inhibited the strain of MRSA and showed a 68.9% reduction in its biofilm. In addition, a methanolic neem oil extract and nimbolide killed cells within in vitro biofilms produced by the Gram-negative carcinogenic pathogen, H. pylori (Guchhait et al., 2022).
The presence of biofilm-forming microorganisms in hospital environments will further pose a serious threat to public health. Hence, there is a need to develop an antimicrobial agent capable of killing biofilm-forming microorganisms or destroying/ penetrating the biofilm produced by microorganisms. There have been several reports on the effectiveness of neem extracts against biofilm-forming microorganisms; however, there is paucity of information on the effectiveness of neem leaf essential oil. Therefore, this study aimed to determine the effectiveness of essential oil from Azadirachta indica against biofilm-producing microorganisms from hospital environments.
2. Materials and Methods
2.1 Collection of Plant Materials and Oil Extraction
Neem leaves were collected from the North gate area of The Federal University of Technology, Akure, Ondo State (Figure 1). Plant leaves were collected before sunrise to prevent plant photo-oxidation; the leaves that had no injury nor chlorosis were sorted out and kept in a clean sack for further work. The plant (commonly referred to as dogoyaro) was authenticated by plant scientists at the Federal University of Technology, Department of Crop Science and Pest Management.
Hydro-distillation method was used for the extraction of essential oil, 100 g of pulverized dried leaf was placed into the thimble and placed in the soxhlet chamber, 500 mL of distilled water was placed in a round bottom flask as the solvent and assembled for soxhlet extractor then the distillation process was begun. After completing the extraction process, the trace of water was removed using a separating funnel, and then extracted essential oil was weighed by using the following equation:

Where: W1 =Sample weight initially placed in the thimble and W2 = weight of oil extracted
2.2 Ethical Approval
Ethical approval was obtained from the University of Medical Sciences Teaching Hospital Complex, Ondo State, with the ethical ID number UNIMEDTHC/028/102. Permission was also sought from the director of health services, The Federal University of Technology, Akure (FUTA), Ondo State, for the collection of swab samples at the University Health Centre. All the information obtained was used for research purposes only.

Figure 1: Azadirachta indica tree
Collection of swab samples and microorganisms identification
Swabs of surgical equipment, hospital doors and some surfaces that the patient frequently touched were collected from the University of Medical Sciences Teaching Hospital, Akure, and the Federal University of Technology, Akure (FUTA) Health Centre. A sterile swab stick was used for the collection of samples from a 10 cm2 area while rotating the swab clockwise and anti-clockwise against the selected area at right angles. Prior to the sample collection, all the swab sticks were moistened with 1.0 ml of sterilized normal saline and covered. Swab samples from selected surfaces were collected according to the reference method of Agbo et al. (2012). Collected swabs were aseptically transferred in an ice pack to the laboratory, Department of Microbiology, Federal University of Technology, Akure, for microbiological analysis, bacteria isolates were identified using colony morphology, growth characteristics on selective and differential media and conventional biochemical tests as described by Fawole and Oso (2004).Fungi were identified based on the colonies’ cultural, morphological and microscopic examination (Hunter & Bamett, 2000).
2.3 Biofilm Formation Assay and Antimicrobial Susceptibility Testing
Bacterial and fungal isolates were cultured at 37oC for 18 hours and 25oC for 36 hours in 1 mL plastic cuvettes; the cuvettes were washed and stained with 0.8 mL of 10 % Crystal Violet. The presence of biofilm was determined by reading the absorbance of the cells attached to the cuvette at 600 nm with acetic acid as the control (blank) as described by Odelola et al. (2023). A bacterial and fungal suspension equivalent to the 0.5 McFarland turbidity standard was prepared for inoculation. Disc and agar well diffusion methods were used to determine the antimicrobial susceptibility profiles of bacterial and fungal isolates. An inhibition zone diameter of each antimicrobial was then measured and was interpreted as resistant (R), intermediate (I) and sensitive (S) (CLSI, 2020).
2.4 Antimicrobial Effects of Neem Oil
The agar well diffusion method was used to determine the antimicrobial potentials of neem oil against drug-resistant biofilm-producing microorganisms, as described by Cheesbrough (2014) and Odelola et al. (2023). Test microorganisms were standardized to 0.5 McFarland turbidity, as previously described by Cheesbrough (2014). Essential oil was reconstituted in 10% Dimethyl sulfoxide (DMSO) to different concentrations (80%, 60%, 40% volume/volume). The sterile petri dishes were filled with 20 mL of Muller Hinton agar and allowed to solidify. Prior to streaking the plates with microbial (fungi and bacteria) culture, 5 mm diameter wells were punched in the medium using a sterile cork borer. The agar plates were streaked with bacterial cultures using sterile swab sticks. Then 100 µL of essential oil was gently dispensed into the agar well made on Muller Hinton agar containing bacterial and fungal cells. Ciprofloxacin (50 µg/mL) and ketoconazole (5 mg/ml) were used as positive controls for bacteria and fungi, respectively, and wells containing DMSO alone were used as negative control. The inoculated plates were incubated at 37oC for 18 hours in bacterial isolates and 25oC for 48 hours in fungal isolates thereafter the diameter of zone of inhibition was measured in mm.
2.5 Determination of Bioactive Components of Essential oil extracted from A. indica Leaf
Agilent Turbo Mass Spectrometer 7890 A GC with chemstation NIST was used for Gas Chromatography-Mass Spectrometry (GC-MS) analysis. The spectrum of the compounds was compared with the database of known compounds stored in the GC-MS library (AOAC, 2018; Oladunmoye, 2019).
2.6 Statistical Analysis
Data obtained was expressed as mean ± Standard Error of Mean. The new Duncan Multiple Range test was used to compare means. A p-value of < 0.05 was considered statistically significant.
3. Results
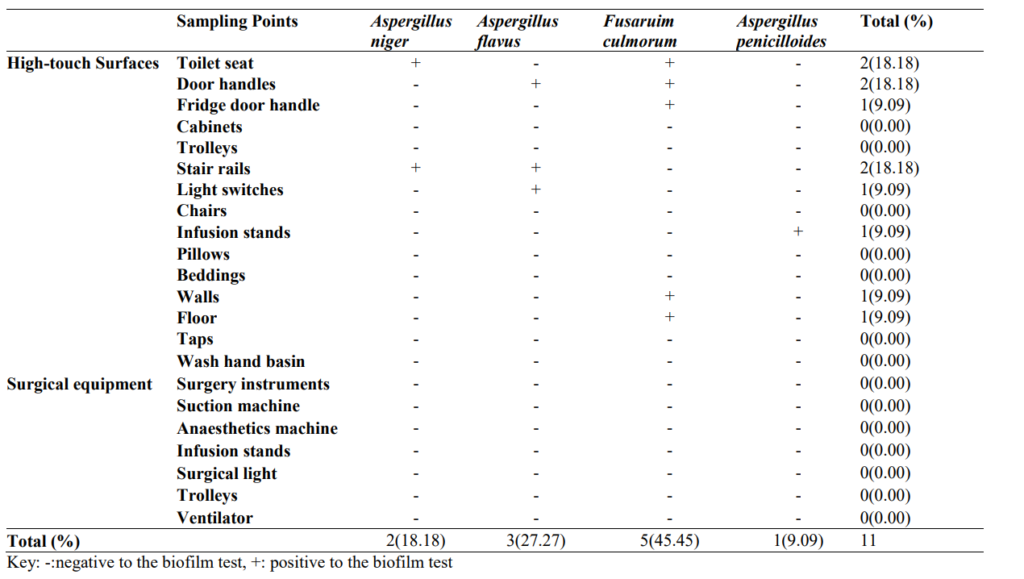
3.1 Occurrence of Biofilm Forming Bacteria in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
Occurrence of biofilm forming bacteria in high-touch surfaces and surgical equipment in selected hospitals in Akure is shown in Table 1. The occurrence of biofilm-producing bacteria in selected hospitals in Akure is 80/172 (46.51%), the presence of biofilm forming bacteria is predominant in stair rails 13/80(16.25%), door handles 10/80(12.50%), beddings 10/80(12.50%), pillows 9/80(11.25%) and cabinets 8/80 (10.00%) while Bacillus subtilis 7/80(8.75%), Escherichia coli 6/80(7.50%), Enterobacter aerogenes 7/80(8.75%), Enterococcus phoeniculicota 6/80(7.50%), Klebsiella pneumoniae 9/80(11.25%) and Pseudomonas aeruginosa 9/80(11.25%) are the most biofilm producers.
3.2 Occurrence of Biofilm Forming Fungi in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
Occurrence of biofilm forming fungi in high-touch surfaces and surgical equipment in selected hospitals in Akure is shown in Table 2. The occurrence of biofilm-producing bacteria in selected hospitals in Akure is 11/96 (11.46%), the presence of biofilm forming bacteria is predominant in toilet seat 2/11(18.18%), door handles 2/11(18.18%), and stair rails 2/11 (18.18%) while Fusaruim culmorum 5/11(45.45%), Aspergillus flavus 3/11(27.27%) and Aspergillus niger 2/11(18.18%) are the most biofilm producers.
Table 1: Occurrence of Biofilm Forming Bacteria in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
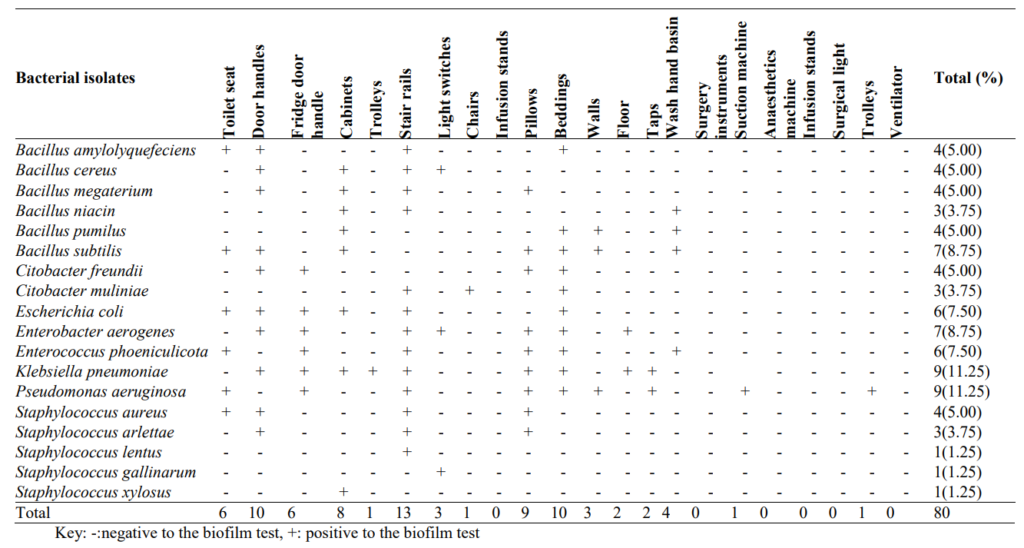
Table 2: Occurrence of Biofilm Forming Fungi in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
3.3 Antibiogram of Biofilm Forming Bacteria in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
An Antibiogram of biofilm-forming bacteria in high-touch surfaces and surgical equipment in selected hospitals in Akure is shown in Table 3. Biofilm-producing bacteria showed higher antibiotic resistance to gentamicin (72.50%), augmentin (52.50%) and cefixime (35%), isolates of C. freundii, C. muliniae, E. coli, E. aerogenes, E. phoeniculicota, K. pneumonia, P. aeruginosa, S. aureus and S. arlettae showed resistance to more than three different antibiotics.
3.4 Antibiogram of Biofilm Forming Fungi in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
An antibiogram of biofilm-forming fungi in high-touch surfaces and surgical equipment in selected hospitals in Akure is shown in Table 4. There is no significant difference in the zones of inhibition of Fusarium culmorum, Aspergillus flavus and Aspergillus niger against ketoconazole. Antifungal inhibition zones ranged from 7.00±0.04 mm (Griseofulvin) to 24.00±0.79 mm (Clotrimazole) against Fusaruim culmorum.
Table 3: Antibiogram of Biofilm Forming Bacteria in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
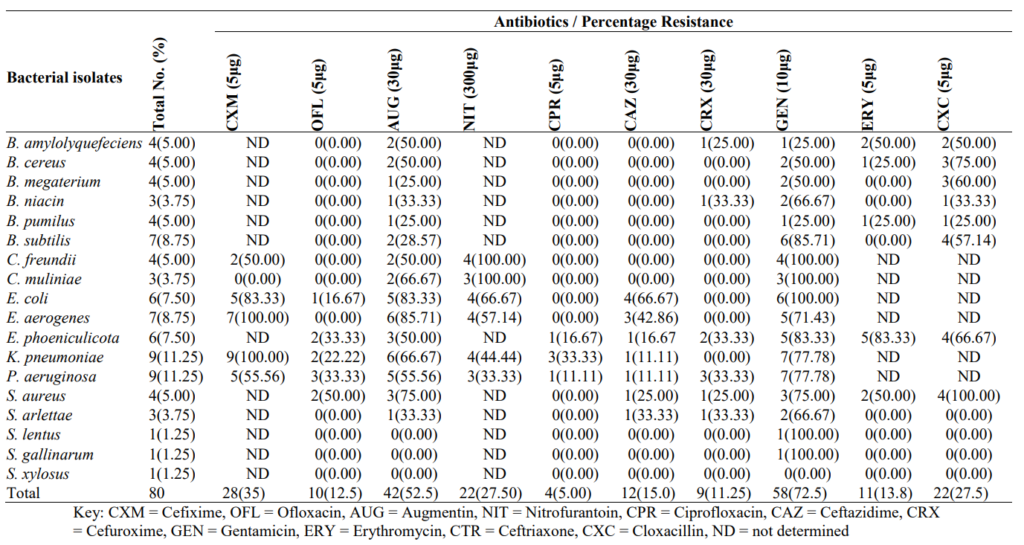
Table 4: Antibiogram of Biofilm Forming Fungi in High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
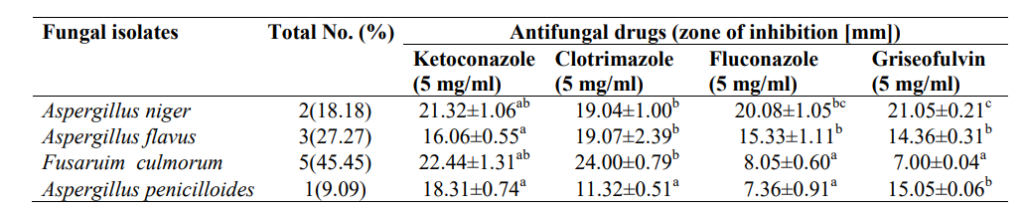
Values are presented as mean ± standard error, values in the same column carrying the same superscript are not significantly different at p<0.05 using the new Duncan Multiple Range test.
3.5 Antibacterial Activity of A. indica Essential oil on Antibiotic-Resistant Biofilm-producing Bacteria Isolated from High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
The antibacterial activity of A. indica essential oil on antibiotic-resistant biofilm-producing bacteria is shown in Table 5. The result revealed significant (p<0.05) differences in the susceptibility of isolates to oil, however, essential oil from A. indica leaf had higher inhibitory effects against B. niacin (20.00±0.00 mm), E. aerogenes (20.00±0.00 mm), P. aeruginosa (24.50±0.11 mm) and S. lentus (20.40±0.11 mm) at a concentration of 80% v/v of oil.
3.6 Antifungal Activity of A. indica Essential Oil on Biofilm-producing Fungi Isolated from High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
The antifungal activity of A. indica essential oil on biofilm-producing fungi isolated from high-touch surfaces and surgical equipment in selected hospitals in Akure is shown in Table 5. Fungal isolates showed significant (p<0.05) higher susceptibility to the oil (80% v/v) than ketoconazole (5 mg/ml). However, the oil had higher inhibition against Aspergillus niger (26.00±0.73mm), Aspergillus flavus (24.02±0.41 mm), Fusarium culmorum (28.19±0.23 mm) and Aspergillus penicilloides (25.44±0.11 mm).
Table 5: Antibacterial Activity of A. indica Essential oil on Antibiotic-Resistant Biofilm-producing Bacteria Isolated from High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
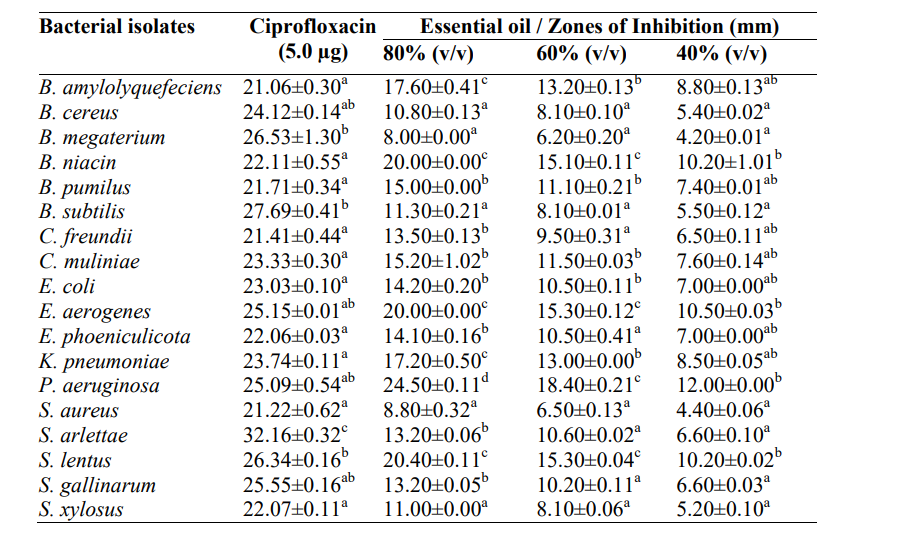
Values are presented as mean ± standard error, values in the same column carrying the same superscript are not significantly different at p<0.05 using the new Duncan Multiple Range test.
Key: v/v – volume per volume, mm – millimetre
Table 6: Antifungal Activity of A. indica Essential Oil on Biofilm-producing Fungi Isolated from High-touch Surfaces and Surgical Equipment in Selected Hospitals in Akure
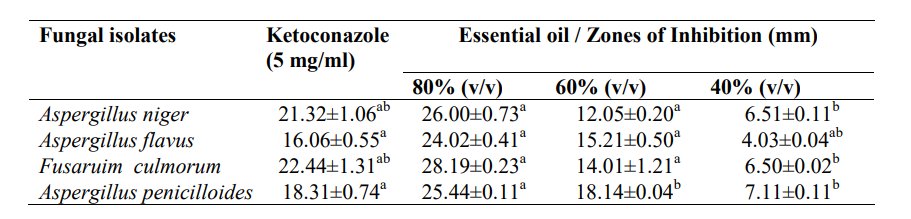
Values are presented as mean ± standard error; values in the same column carrying the same superscript are not significantly different at p<0.05 using the new Duncan Multiple Range test.
Key: v/v – volume per volume, mm – millimetre
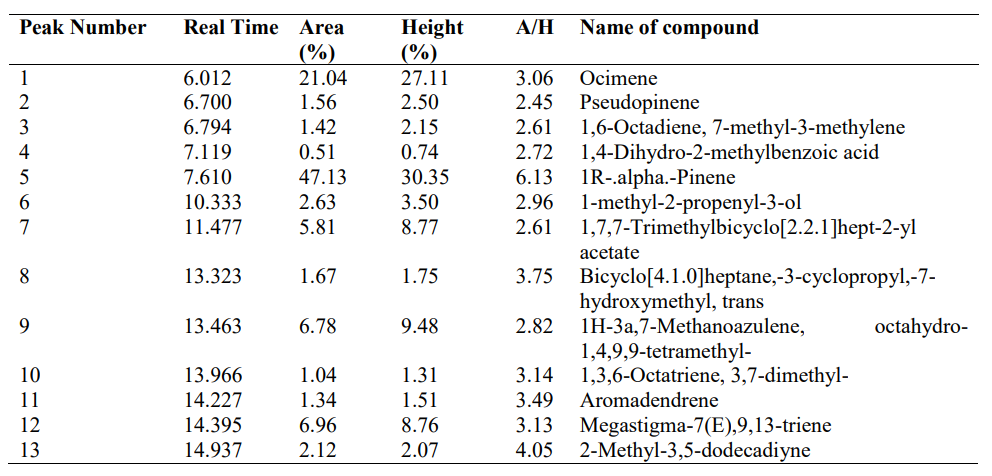
3.7 Gas Chromatography-Mass Spectrophotometry Spectra of A. indica Essential oil and Bioactive Compounds
Figure 2 and Table 7 show the Gas Chromatography-Mass spectrophotometry spectra of A. indica essential oil and bioactive compounds. 1R-.alpha.-Pinene (47.13%) and Ocimene (21.04%) are the most abundant compounds in the essential oil of A. indica leaf.
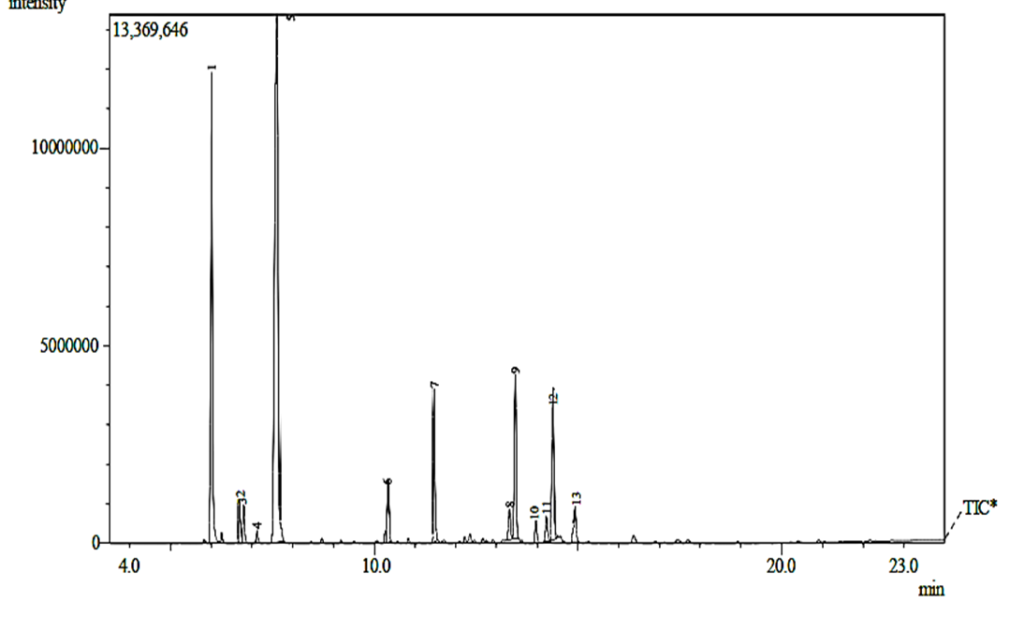
Figure 2: Gas Chromatography-Mass Spectrophotometry Spectra of A. indica Essential oil
Table 7: Organic Compounds Abundantly Present in A. indica Leaf Essential Oil
4. Discussion
Hospital contained antibiotics (ATB), disinfectants, metabolized drugs and microorganisms from hospitalized patients. These made hospitals significant and important contributors to the spread and development of antibiotic resistance in the environment. Studies have demonstrated the presence of microorganisms in hospital environments and medical equipment; these are also susceptible to microbial adhesion and biofilm formation (Sharma et al., 2023).
Also, poor hygienic condition of the hospital environment could have been responsible for the higher occurrence of spore-forming microorganisms like Bacillus spp., Aspergillus niger and Aspergillus flavus isolated from study areas. However, among the bacterial isolated, the biofilm producers were predominantly Bacillus subtilis, Escherichia coli, Enterobacter aerogenes, Enterococcus phoeniculicota, Klebsiella pneumoniae and Pseudomonas aeruginosa. This corroborates the studies that stated that the most frequently found bacteria in a biofilm are Pseudomonas aeruginosa, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Streptococcus viridans, Staphylococcus aureus, and Enterococcus faecalis (Chen et al., 2013; Sharma et al., 2023).Also, 70% of all bacterial infections are linked with biofilm and are both device-related (e.g. surgical equipment) and non-device-related (e.g. hospital bedding, tables, and chairs) (Jamal et al., 2018). The occurrence and variations in the presence of biofilm-producing microorganisms in high-touch surfaces and surgical equipment could be attributed to variations in surface-to-surface, bacterial adhesion, hospital practices, age of materials in the hospital environment and the different surface adaptability.
Infections caused by biofilm-producing microorganisms are now a major concern in the healthcare industry because of their higher tendency to resist all the existing antimicrobials (Sharma et al., 2023). The biofilm-forming bacteria were highly resistant to cefixime, augmentin and gentamicin; studies have shown that bacteria that inhabit biofilms show a 10 to 1000-fold increase in drug resistance (Yang et al., 2021; Sharma et al., 2023), which may be responsible for the occurrence of resistance in this study. In this study, the higher resistance of biofilm-forming bacteria to beta-lactam drugs (cefixime, and augmentin) and gentamicin could be that the antibiotics were the most used in the hospitals used for this study. This resistance could have been formed by delayed or inadequate diffusion of the antimicrobial agents through the biofilms, varied growth rate of the biofilm microbial organisms, and other functional alterations that are responsible for the development of antimicrobial resistance. Bacteria in the planktonic state are known to use either enzymes, efflux pumps, or mutations as a means to evade drugs and develop resistance, as reported by (Sharma et al. (2019) and Dan et al. (2023).
The antifungal drugs (fluconazole and griseofulvin) showed a reduced zone of inhibition against Fusarium culmorum and Aspergillus penicilloides in this study. Within a biofilm, a number of distinct components work together to mitigate or completely prevent the effectiveness of drugs by fungi, which further drives resistance. Strategies are decreased penetration of the drug (Liu et al., 2022), biofilm’s modification of chemical microenvironment (Dan et al., 2023), and differentiation to spore formation (Liu et al., 2022). Similar rates have been reported from tertiary hospitals in South-West Nigeria by Ochada et al. (2015) and Odelola et al. (2023), while another study by Oluwafemi et al. (2018), reported higher susceptibility rates. This infers that the antibiogram pattern of biofilm-forming microorganisms varies from place to place depending on the types of antibiotics and hospital hygienic practices, as well as the adaptation of the isolates.
Biofilm control is a difficult problem to solve. The frequency and severity of infections may grow, which would increase infection-related mortality and morbidity if biofilms are not properly combated (Algburi et al., 2017). Because of the grave danger of antibiotic resistance posed by biofilm microorganisms, it is vital to find effective treatment options for biofilm-associated infections (Odelola et al., 2023). In this study, the essential oil of A. indica leaf showed higher inhibitory effects against biofilm-forming B. niacin, E. aerogenes, P. aeruginosa, S. lentus, Aspergillus niger, Aspergillus flavus, Fusaruim culmorum and Aspergillus penicilloides. Other extracts of neem were reported to be more consistently effective at prohibiting bacterial growth and at targeting biofilm-grown cells than many other herbal extracts and are, therefore, worth pursuing as a source for drug discovery (Saleem et al., 2018). Different studies have established the antimicrobial effects of A. indica against biofilm-forming organisms like Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans and Candida albicans (Singh et al., 2021; Hosny et al., 2021; Tasanarong et al., 2021). However, there is paucity of information on the inhibitory effects of essential oil from neem leaf against biofilm-forming organisms which was established in this study. The inhibitory effects shown by the oil could be because of the concerted effects of different bioactive components present in the oil. Additionally, synergistic interactions between the many constituents found within the oil may provide a benefit over a single isolated ingredient; this may explain the efficacy of lower doses of neem oil against biofilm formers (Zihadi et al., 2019).
In this study, different bioactive compounds were identified in the oil of A. indica leaf, among which the most abundant compounds were 1R-.alpha.-Pinene and Ocimene. 1R-α-Pinene is a volatile monoterpene with antimicrobial activities, α-Pinene is an organic compound of the terpene class. An alkene and it contains a reactive four-membered ring. It is found in the oils of many species of coniferous trees. It was evidenced that its effectiveness is directly linked to its concentration and mechanism of action by inhibition of efflux pump in bacteria, the interaction with certain bacterial strains and in some cases, the concomitant action of antibiotics, acting in the latter case as a synergist potentiating the drug (Milar et al., 2022). This compound, when separated, could be used as an alternative for combatting biofilm-forming bacteria. The ocimenes are a group of monoterpenes found in plants and fruits and have been known to have mild antimicrobial activity. They have a pleasant odour and are used in perfumes for their herbal scent. Ocimenes are unstable and are easily oxidized in air; the mechanism of their antibacterial activities is membrane disruption. These compounds reported in this study have been reported to have antimicrobial, antiparasitic, antioxidant, preservative and immunomodulatory effects. Also, these compounds have been reported in the literature to have greater potential for biofilm eradication, persister cell damage, disruption of EPS structural components, and prevention of quorum sensing (Lahiri et al., 2021).
5. Conclusion
This study has revealed that high-touch surfaces and surgical equipment were contaminated with microorganisms in the study area among which Bacillus subtilis, Escherichia coli, Enterobacter aerogenes, Enterococcus phoeniculicota, Klebsiella pneumoniae and Pseudomonas aeruginosa were predominantly biofilm producers, these bacteria showed varied degrees of resistance to different antibiotics and essential oil of A. indica leaf showed inhibitory effects against biofilm forming antibiotic-resistant bacteria in this study. The chemical compounds present in the oil mean that it could also serve as antimicrobial and could be used as a disinfectant for routine cleaning in the hospital environment to control biofilm-forming microorganisms.
Conflict of Interest
The Authors declared no conflict of interest.
References
Agbo, H. A., Envuladu, E. A., Adah, U. G. and Zoakah, A. I. (2012). An assessment of toilet facilities in secondary schools in Jos North Local Government Area of Plateau State. Greener Journal of Educational Research, 2 (4): 091 – 094.
Algburi, A., Comito, N., Kashtanov, D., Dicks, L. M. T. and Chikindas, M. L. (2017). Control of Biofilm Formation: Antibiotics and Beyond. Applied Environmental Microbiology, 83: e02508-16.
AOAC (2018). Association of Official Analytical Chemists Official Methods of Analysis. (21st edition.). W. Hortuntzed (Edition), Washington
Borges A., Abreu A. C., Dias C., Saavedra M. J., Borges F., Simões M. (2016). New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules, 21 (7). 10.3390
Braga T. M., Rocha L., Chung T. Y., Oliveira R. F., Pinho C. and Oliveira A. I. (2020). Biological Activities of Gedunin-A Limonoid from the Meliaceae Family. Molecules, 25 (3): 10.3390.
Brown, H. L., Reuter, M., Hanman, K., Betts, R. P. and van Vliet, A. H. (2015). Prevention of biofilm formation and removal of existing biofilms by extracellular DNases of Campylobacter jejuni. PLoS ONE, 10: e0121680.
Cheesbrough, M. (2014). District Laboratory Practice in Tropical Countries. 4th Edition., Cambridge University Press, Cambridge, UK., ISBN-13:9781139449298. 50: 165-176
Chen, M., Yu, Q. and Sun, H. (2013). Novel strategies for the prevention and treatment of biofilm-related infections. International Journal of Molecular Sciences, 14: 18488–18501.
Committee for Clinical Laboratory Standards (CLSI), (2020). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Seventh Informational Supplement. CLSI document M100-S27 Wayne, PA: ISBN 1-56238-898-3. 34(1): 50-98
Dan, B., Dai, H., Zhou, D., Tong, H. & Zhu, M. (2023). Relationship Between Drug Resistance Characteristics and Biofilm Formation in Klebsiella pneumoniae Strains. Infection and Drug Resistance, 16: 985–998.
Fawole, M. O. and Oso, B. A. (2004). Characterization of bacteria: Laboratory Manual of Microbiology. 4th Edition, Spectrum Book Ltd., Ibadan, Nigeria, 24-33
Guchhait, K. C., Manna, T., Barai M., Karmakar M., Nandi S. K. and Jana D. (2022). Antibiofilm and Anticancer Activities of Unripe and Ripe Azadirachta indica (Neem) Seed Extracts. BMC Complement Medicine and Therapy, 22 (1): 42.
Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Trivedi, P. (2016). Biofilm, pathogenesis and prevention–a journey to break the wall: A review. Archives of Microbiology, 198, 1–15.
Hosny N. S., El Khodary S. A., El Boghdadi R. M. and Shaker O. G. (2021). Effect of Neem (Azadirachta indica) versus 2.5% Sodium Hypochlorite as Root Canal Irrigants on the Intensity of Post-operative Pain and the Amount of Endotoxins in Mandibular Molars with Necrotic Pulps: a Randomized Controlled Trial. International Endod Journal, 54 (9): 1434–1447.
Hunter, B. B. and Berneth, H. L. (2000). Illustrated Genera Imperfect Fungi. (5th ed). Burgess, New York, USA, 230–241.
Jamal, M., Ahmad, W., Andleeb, S., Jalil, F., Imran, M., Nawaz, M. A., Hussain, T., Ali, M., Rafiq, M. and Kamil, M. A. (2018). Bacterial biofilm and associated infections. Journal of China Medical Association, 81: 7–11.
Jiang, Y., Geng, M. and Bai, L. (2020). Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms. 8: 1222.
Kaur R., Tiwari A., Manish M., Maurya I. K., Bhatnagar R. and Singh S. (2021). Common Garlic (Allium sativum L.) Has Potent Anti-Bacillus Anthracis Activity—Journal of Ethnopharmacology, 264: 113230.
Lahiri, D., Nag, M., Dutta, B., Mukherjee, I., Ghosh, S. & Dey, A. (2021). Catechin as the Most Efficient Bioactive Compound from Azadirachta indica with Antibiofilm and Anti-Quorum Sensing Activities against Dental Biofilm: an In Vitro and In Silico Study. Applied Biochemistry and Biotechnology, 193 (6): 1617–1630.
Liu, Q., Yin, L., Zhang, X., Zhu, G., Liu, H., Bai, F., Cheng, Z., Wu, W. & Jin, Y. (2022). Reversion of Ceftazidime Resistance in Pseudomonas aeruginosa under Clinical Setting. Microorganisms, 10: 2395
Maillard, J. and Centeleghe, I. (2023). How biofilm changes our understanding of cleaning and disinfection. Antimicrobial Resistance & Infection Control, 12:95 – 115
Nagini S., Nivetha R., Palrasu M., Mishra R. (2021). Nimbolide, a Neem Limonoid, Is a Promising Candidate for the Anticancer Drug Arsenal. Journal of Medical Chemistry, 64 (7): 3560–3577.
Ochada, N. S., Nasiru, I. A., Thairu, Y., Okanlowan, M. B. and Abdulakeem, Y. O. (2015). Antimicrobial susceptibility pattern of urinary pathogens isolated from two tertiary hospitals in Southwestern Nigeria. African Journal of Clinical and Experimental Microbiology, 16(1): 12–22.
Odelola, O. S., Oyetayo, V. O., Ogundare, A. O., Omoya, F. O. and Ajayi, O. E. (2023). Antibacterial Activity of Zea Mays Silks and Husks Crude Extract on Biofilm Producing Multi-Drug Resistant Bacteria from Urinary Catheters. International Journal of Pathogen Research, 12(2): 37-51
Oladunmoye, M. K. (2019). Characterization of Organic Compounds in Aframomum melegueta K. Schum Using GC-MS. Medicinal and Aromatic Plants,8(2): 1-8
Oluwafemi, T. T., Akinbodewa, A. A., Ogunleye, A. and Adejumo, O. A. (2018). Urinary tract infections and antibiotic sensitivity pattern of uropathogens in a tertiary hospital in South West, Nigeria. Sahel Medical Journal, 21(1): 18–22.
Paharik, A. E. and Horswill, A. R. (2016). The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiological Spectra, 4: 7 -21
Saleem S., Muhammad G., Hussain M. A. and Bukhari S. N. A. (2018). A Comprehensive Review of Phytochemical Profile, Bioactives for Pharmaceuticals, and Pharmacological Attributes of Azadirachta indica. Phytotherapy Research, 32 (7): 1241–1272.
Sharma, D., Misba, L. and Khan, A.U. (2019). Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resistance and Infection Control, 8: 76.
Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. (2023). Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms, 11: 1614
Singh V., Roy M., Garg N., Kumar A., Arora S. & Malik D. S. (2021). An Insight into the Dermatological Applications of Neem: A Review on Traditional and Modern Aspect. Recent Advances in Antiinfection Drug Discovery, 16 (2): 94–121.
Tasanarong T., Patntirapong S. and Aupaphong V. (2021). The Inhibitory Effect of a Novel Neem Paste against Cariogenic Bacteria. Journal of Clinical and Experimental Dentition, 13 (11), e1083–e1088.
Vandana Das, S. (2021). Structural and mechanical characterization of biofilm-associated bacterial polymer in the emulsification of petroleum hydrocarbon. 3 Biotechnology, 11: 239.
Yang, J., Yao, J. L., Wu, Z. Q., Zeng, D. L., Zheng, L. Y., Chen, D., Guo, Z. D. and Peng, L. (2021). Current opinions on the mechanism, classification, imaging diagnosis and treatment of post-traumatic osteomyelitis. Chin. Journal of Traumatology, 24: 320–327.
Zihadi M. A. H., Rahman M., Talukder S., Hasan M. M., Nahar S. and Sikder M. H. (2019). Antibacterial Efficacy of Ethanolic Extract of Camellia sinensis and Azadirachta indica Leaves on Methicillin-Resistant Staphylococcus aureus and Shiga-Toxigenic Escherichia coli. Journal of Advance Veterinary Animals and Research, 6 (2): 247–252.
About this Article
Cite this Article
APA
Omoya, F. O., Salami, F. F. & Ajayi, K. O.(2025). Azadirachta indica Leaf Essential Oil as Control of Biofilm-Producing Microorganisms from Hospital Environment. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Omoya, F. O., Salami, F. F. And Ajayi, K. O. 2025. “Azadirachta indica Leaf Essential Oil as Control of Biofilm-Producing Microorganisms from Hospital Environment.” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
10 November 2024
Accepted
15 January 2025
Published
4 February 2025
Corresponding Author Email: : ooolusola-makinde@futa.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.