Omoya F. O. 1 Ajayi K. O. 2 Fasanya K. 3 Oladele O. 4 Famuyiwa V. 5 & Ejuwa K. 6
1Department of Medical Laboratory Science, Federal University of Technology, Akure, Nigeria
2Department of Microbiology, School of Life Sciences, Federal University of Technology, Akure, Nigeria
3Department of Natural Sciences (Microbiology), Faculty of Pure and Applied Sciences, Precious Cornerstone University, Ibadan
*Corresponding Author Email:ajayikehinde@pcu.edu.ng
Abstract
Malaria, caused by Plasmodium parasites, remains a major global health concern, with increasing drug resistance necessitating alternative treatments. This study evaluates the effects of essential oils from Azadirachta indica leaves and Picralima nitida seeds on liver and kidney function indices in Plasmodium berghei (NK-65)-infected mice. Essential oils were extracted via hydrodistillation and assessed for oral acute toxicity, parasite load, haematological parameters, and organ function. Toxicity tests at doses up to 5000 mg/kg body weight showed no adverse effects, indicating a promising safety profile. At 300 mg/mL, both oils reduced parasitaemia to 0% within 72 hours, whereas untreated mice exhibited 9.60% parasitaemia. Treated mice showed significant improvements in packed cell volume (PCV: 34.9%–44.5%) and white blood cell count (0.6–0.8 ×10⁹/L), compared to lower values in the untreated group (PCV: 12.2%, WBC: 0.1 ×10⁹/L). Liver function markers, including AST, ALT, ALP, total protein, bilirubin, and albumin, aligned closely with chloroquine-treated controls, indicating maintained hepatic health. Kidney function markers (sodium, potassium, chloride, urea, creatinine) were also comparable to the control group. These findings suggest that A. indica and P. nitida essential oils are non-toxic and effective in mitigating malaria-induced organ dysfunction. Their biochemical effects mirror those of chloroquine, supporting their potential as alternative antimalarial agents. Further studies should explore clinical trials, mechanism elucidation, and combination therapy efficacy.
Keywords: Antibiotic Resistance, Beef, Clostridium perfringens, Microbial Risk Assessment, 16S rRNA.
1. Introduction
Malaria remains a persistent threat to human health and significantly impacts economic growth and development in regions where it is prevalent, mostly in sub-Saharan Africa. It has also played a significant role throughout human history. Malaria is a major parasitic disease spread by female Anopheles mosquitoes, which transmit the sporozoite stage of the pathogen to humans during blood-feeding. Nigeria accounted for the highest proportion (27%) of global malaria cases; notwithstanding, significant progress has recently been made in controlling and eliminating malaria in many endemic areas including Nigeria, particularly with the introduction of vaccines (Yusuf & Aliyu-Paiko., 2020; WHO, 2024). However, Plasmodium resistance to current antimalarial drugs, mosquito resistance to insecticides, and climate change continue to be significant obstacles to the elimination of malaria (Hanboonkunupakarn et al., 2022). Climate change exacerbates malaria prevalence by altering temperature and rainfall patterns, which expand mosquito breeding habitats and increase transmission seasons in previously unaffected areas. Therefore, studies are still being conducted to identify new sources of antimalarial drugs, with medicinal plants being a promising avenue. For instance, the discovery of artemisinin from Artemisia annua revolutionised malaria treatment and remains a cornerstone of current antimalarial therapies, highlighting the potential of medicinal plants in addressing drug resistance and improving global health outcomes (Alghamdi et al., 2024). Numerous antimalarial drugs, especially those used for prophylaxis, may induce adverse effects such as gastrointestinal disturbances, headaches, dizziness, and, in some cases, hepatotoxicity or visual disturbances, which curtail their widespread use (Figueroa-Romero et al., 2022). Prioritising drugs effective against the parasite and well tolerated by diverse populations, including vulnerable groups like pregnant women and children, is paramount (WHO, 2024). The essential oils from Picralima nitida and Azadirachta indica offer a promising alternative, as they have shown low toxicity and may be safer for use in these vulnerable populations. Malaria parasites undergo complex life cycles involving both the human host and the mosquito vector. Existing drugs primarily target the blood stage, leaving other stages susceptible to resurgence (Munir, 2024). Identifying compounds effective against multiple parasite life cycle stages, including liver and mosquito stages, is essential to prevent relapses and interrupt transmission (Rosado-Quiñones et al., 2024).
Picralima nitida (P. nitida) belongs to the Hunterieae tribe of the Apocynaceae family and is extensively found in the high deciduous forests of West-Central Africa (Kebede, 2023). Traditionally, it has been used in local medicine to treat various ailments, including malaria, which supports its potential as an effective antimalarial based on both ethnobotanical evidence and emerging scientific research. In West African traditional medicine, P. nitida is used in a broad variety of ways. Herbalists employ different components of the plant, such as the leaves, seeds, stem bark, and roots, to cure malaria, hypertension, fever, jaundice, and gastrointestinal ailments (Evbuomwan et al., 2023).Azadirachta indica, commonly known as Neem, has gained global attention in recent years owing to its diverse medicinal properties. It is widely recognised for its use in treating skin disorders, supporting oral health, and as a natural insect repellent, with ongoing research into its potential as an antimalarial agent (Singh et al., 2021). The tree has been extensively utilised in naturopathy, Unani, and homoeopathic medicine, emerging as a guiding principle in contemporary medical practices (Shaheen & Sarwar, 2024). Additionally, Neem encompasses a broad spectrum of biologically active compounds, with over 150 isolated from various parts and categorised into isoprenoids and non-isoprenoids, including proteins and carbohydrates (Faisal et al., 2023). Research on these plants continues to explore their potential in overcoming malarial drug resistance. Specifically, studies have focused on examining the safety and effects of P. nitida seed and A. indica leaf oils on liver and kidney functions in P. berghei NK-65 infected mice, highlighting their therapeutic potential. The liver is the primary organ affected by malaria parasites, though the disease can also impact other organs, including the kidneys, lungs, and brain. In severe cases, malaria can lead to organ failure, respiratory distress, and cerebral malaria, highlighting the broader systemic effects of the infection (Okagu et al., 2022). Severe malaria can cause disease in the glomeruli, tubules, and interstitial regions, potentially leading to kidney complications such as acute kidney injury (AKI) (Siagian, 2022). Studies have shown that approximately 10–20% of severe malaria cases develop AKI, underscoring the significant renal impact of the infection (Batte et al., 2023). The sporozoites of malaria enter the liver and infect hepatocytes (Mehlhorn, 2023). The parasites replicate asexually within liver cells until they mature into schizonts. Merozoites then enter the bloodstream through infected hepatocyte rupture. Infected hepatocytes burst, causing liver injury (Frischknecht, 2023). Damage to liver cells can elevate the enzymes responsible for liver function, particularly transaminase enzyme activity, and lead to morphological changes such as hepatomegaly (Martins et al., 2024; Muganda, 2022). The essential oils tested in this study, however, appear to mitigate these effects, as they demonstrated potential in stabilising liver enzyme levels and reducing liver enlargement in infected mice.
2. Material and Methods
2.1 Plant Materials and Preparation of Picralima Nitida and Azadirachta Indica Essential Oils
Seeds of P. nitida and fresh leaves of A. indica were identified by a plant expert at the Federal University of Technology, Akure, and carefully collected by plucking from the tree while wearing gloves. The trees were located on the premises of the Federal University of Technology, Akure, Ondo State, Nigeria. The seeds of P. nitida were obtained from the Odo Ipetu market in Akure, Ondo State capital, a local marketplace known for its variety of medicinal plants, making it a relevant source for this study. One hundred grams of dried seeds and fresh leaves were placed in a 2000 mL round-bottom flask, and 600 mL of distilled water was added, resulting in a plant-to-water ratio of 1:6. This ensured that the entire sample was immersed for extraction. The round-bottom flask was placed on a heating mantle and connected to the Clevenger apparatus, which is preferred for essential oil extraction due to its efficiency in distilling volatile compounds while separating the essential oils from the plant material. Water was allowed to flow into the condenser to facilitate the distillation process. While boiling, the volatile oils were carried along with the steam into the graduated distillate receiving tube, and excess water returned to the flask. A layer of solvent, a mixture of dichloromethane and diethyl ether, was added to the distillation arm. The essential oils dissolved in the organic solvent mixture in the graduated distillate receiving arm. Heating was continued for about 5 hours, a duration optimised based on prior studies to ensure thorough extraction of essential oils. The assembly was then allowed to cool. The essential oil was obtained, weighed, and stored in airtight half‑ounce amber bottles in the refrigerator at 4°C to preserve its integrity until it was used for the experiment.


2.2 Animals Used for this Study
Forty-four (44) inbred male and female (non-pregnant) albino mice, weighing between 16–21 g, were purchased from the Institute of Malaria Research Laboratory (IMRAT) of the Department of Basic Medical Science, University of Ibadan, Oyo State, Nigeria. This specific strain of albino mice was chosen due to its genetic consistency, which ensures reliable results in experimental studies, while the weight range of 16–21 g was selected to ensure that the mice were at an appropriate age and size for handling and treatment. The animals were housed in standard laboratory cages for a 14-day acclimatisation period, which is crucial for reducing stress and ensuring that the mice adapted to their new environment before experimental procedures. They were maintained under good laboratory practices, with a 12-hour light/dark cycle, a uniform temperature of 25–28°C, and free access to standard finisher pellets and clean, distilled water. This period helped to stabilise the mice’s physiological state, ensuring the reliability of the experimental results. The laboratory animals were used in accordance with laboratory practice regulations and the principles of humane laboratory animal care as documented by Zimmermann (1983).
2.3 Acute Toxicity Test
The LD₅₀ was calculated using Karber’s method, which is preferred for its simplicity and accuracy in determining the median lethal dose from dose–response data, particularly in cases where the number of dose levels is limited. This method is widely used in toxicological studies and provides reliable results with fewer animals compared to alternative LD₅₀ calculation methods. It involves the administration of different doses of the test substance to various groups of five animals each (Dong et al., 2024). The first group of animals was administered the vehicle in which the test substance was dissolved or diluted; in this study, this was distilled water. From the second group onward, different doses of the test substance were administered. The animals in each group received specific doses, with the dose increment progressing from group to group. The experimental mice were grouped into four groups. The first group was administered a dose of 5000 mg/kg of the essential oil, prepared by dissolving 0.6 mL of the oil in 0.4 mL of water. The second group was administered 2000 mg/kg and the third group 1000 mg/kg, prepared by dissolving 0.25 mL of oil in 0.75 mL of distilled water. The mice were then observed for signs of toxicity and mortality.
The LD₅₀ is calculated using the formula:
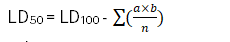
Where:
n = group population
LD₅₀ = median lethal dose
LD₁₀₀ = least dose required to kill 100%
a= dose difference
b= mean mortality
2.4 Collection of Donor Mice, Parasite Strain, Preparation of Parasite Inoculum and Mice Infection
Mice infected with Plasmodium berghei NK-65 (P. b) were collected from the Institute for Advanced Medical Research and Training, University College Hospital (UCH), Ibadan, Oyo State, Nigeria. In vivo antimalarial testing in mice was conducted using a chloroquine-sensitive strain of P. berghei NK-65. The parasite stock was maintained by continuous re-infection in the mice. Donor P. berghei-infected mice were rendered unconscious using chloroform, and blood was collected by cardiac puncture into a heparinised tube. Heparinised blood containing approximately 30% parasitaemia (i.e. 30% of the erythrocytes are parasitised) was diluted with 5 mL of phosphate buffer solution (PBS) at pH 7.2 so that each 0.2 mL contained approximately 1 × 10⁷ infected erythrocytes. An aliquot of 0.2 mL (2 × 10⁷ parasitised erythrocytes) of this suspension was injected intraperitoneally into experimental mice. Standard single-use disposable sterile syringes were used. Each animal received an inoculum of about 10 million parasites per kilogram body weight, which is expected to produce a steadily rising infection in mice (David et al., 2004).
2.5 Experimental Design
Forty-five (45) mice were selected and divided into nine (9) groups, with five (5) mice per group, to ensure statistically significant results while minimising animal usage. This sample size per group is commonly used in preclinical studies to balance statistical power and ethical considerations, allowing for meaningful comparisons while keeping the number of animals used to a minimum.
- Group A – Infected and treated with oil 300 mg/mL of Azadirachta indica
- Group B – Infected and treated with oil 100 mg/mL of Azadirachta indica
- Group C – Infected and treated with oil 300 mg/mL of Picralima nitida (abere)
- Group D – Infected and treated with oil 100 mg/mL of Picralima nitida (abere)
- Group E – Uninfected and treated with oil of Azadirachta indica
- Group F – Uninfected and treated with oil of Picralima nitida (abere)
- Group G – Infected and untreated
- Group H – Infected and treated with an antimalarial drug
- Group I – Uninfected and untreated
Rane’s test of established infection, as stated by Rukayyah et al. (2015), was adopted in this study. In the curative assay, mice were infected and left for 72 hours before treatment. Body weight and temperature were monitored during the infection period for 72 hours, as these are key indicators of general health and physiological response to infection. Changes in body weight and temperature can provide early signs of infection severity, including potential organ dysfunction, and are commonly used to assess the effects of treatments or interventions in preclinical studies. Groups G and I received 5 mL/kg of sterile distilled water. Groups A, B, C, D, E and F were administered the essential oil orally every day for 72 hours. Group H was administered 5 mg/kg/day. Thick and thin blood smears from tail-tip cuts were examined for parasites at 24, 48, and 72 hours during the treatment period. Average parasitaemia and the percentage chemo-suppression were calculated using the following formulas:
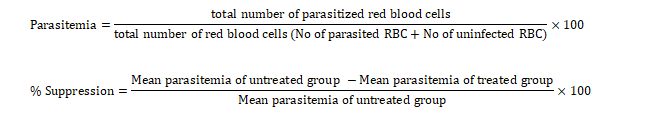
Finally, the percentage parasitaemia suppression of the essential oil was compared with respect to the controls. Parasitaemia suppression was calculated using the method of Hilou et al. (2006), which involves determining the reduction in parasitaemia by comparing the number of parasitised erythrocytes in treated animals to those in untreated controls, expressed as a percentage of suppression relative to baseline levels.
2.6 Determination of Mean Weight and Temperature
The weight of the mice was measured using a digital weighing balance with a precision of ±0.01 g, and the average weight was calculated. The temperature of the mice was measured regularly using a digital thermometer by placing it in the mouth.
2.7 Haematological Assay
Blood samples were collected from the mice by anaesthetisation, and cardiac puncture was used to withdraw blood. The blood collected was carefully transferred into ethylenediaminetetraacetic acid (EDTA) bottles for haematological assays (Cheesbrough, 2014). Haematological tests such as packed cell volume, white blood cell (WBC) count, red blood cell (RBC) count, haemoglobin (HGB), haematocrit (HCT), mean cell volume (MCV), mean cell haemoglobin (MCH), and mean cell haemoglobin concentration (MCHC) were performed according to the methods described by Cheesbrough (2014). Control samples were included in each assay to ensure quality assurance and the accuracy of the results.
2.8 Determination of Liver and Kidney Function Enzymes
Blood from cardiac puncture was dispensed into lithium heparin bottles and centrifuged at 1372 g for 10 minutes, after which the clear serum was aspirated. The serum was then thawed and assayed for the levels of aspartate transaminase (AST), alanine transaminase (ALT), total proteins, total bilirubin and albumin using the method of Johnson and Modo (2024). The activity of alkaline phosphatase (ALP) was assayed by the method of Yusuf and Aliyu-Paiko (2020), as outlined in Randox kits (UK), which were validated for use in this experimental context to ensure accurate and reliable results. Kidney function parameters such as urea, electrolytes (sodium, phosphate) and creatinine were also assayed.
2.9 Data Analysis
Data were analysed using IBM Statistical Package for the Social Sciences (IBM-SPSS) version 20. Differences in parameters were compared using Duncan’s New Multiple Range Test at p < .05. Other data were subjected to two-way analysis of variance (ANOVA), and means were compared using Duncan’s New Multiple Range Test, with p < .05 taken as significant.
3. Results
3.1 Acute Toxicity Tests
The oral acute toxicity properties of essential oils from Azadirachta indica leaves and Picralima nitida seeds are presented in Table 1. Oral acute toxicity tests are critical in evaluating the potential therapeutic applications of these essential oils, as they help assess the safety profile, determine safe dosage levels, and identify any harmful effects that could limit their use in treatments. The oil demonstrated an LD₅₀ > 5000 mg/kg body weight, as no mortality or physical and behavioural changes were observed in animals administered the oil up to 5000 mg/kg body weight throughout the 7 days of observation. An LD₅₀ greater than 5000 mg/kg indicates a high safety margin, suggesting that the oil is unlikely to cause acute toxicity at therapeutic doses. This finding is significant for its potential use in humans, as it implies a minimal risk of severe adverse effects at commonly used dosages.
3.2 General Appearance and Behavioural Observations in Mice Administered with Essential Oils from Azadirachta indica and Picralima nitida during the Oral Acute Toxicity Study
The physical appearance and behavioural observations of mice administered with essential oils from Azadirachta indica leaves and Picralima nitida seeds are shown in Tables 2a and 2b, respectively. These specific behavioural and physical parameters were chosen because they provide valuable insights into the general health and well-being of the animals, helping to detect signs of toxicity, distress, or adverse effects following administration of the essential oils. The mice generally showed no changes in behavioural characteristics or food intake. No drowsiness, sedation, or mortality was observed. The absence of these adverse effects indicates that the oils are well tolerated at the administered doses, making them safe as potential therapeutic agents.
3.3 General Appearance and Behavioural Observations in Infected Mice Treated with Essential Oil from Azadirachta indica and Picralima nitida
The physical appearance and behavioural observations of infected mice treated with essential oils from Azadirachta indica leaves and Picralima nitida seeds are shown in Tables 3a and 3b, respectively. These observations demonstrate the efficacy of the oils, as there were no adverse effects or anomalies observed. The mice generally showed no changes in behavioural characteristics or food intake. No drowsiness, sedation, or mortality was observed. This lack of change underscores the safety of the oils at the administered dosages, as it indicates that the treatments did not interfere with the animals’ normal physiological or behavioural functions, thereby supporting their potential use as safe therapeutic agents. An increase in body weight was observed in the groups that were uninfected but treated with the essential oils at a high concentration. A reduction in body weight was observed in mice treated with the essential oil from Azadirachta indica leaves at a high concentration. This weight reduction could be due to the oil’s metabolic effects or mild toxicity at higher doses. While this finding does not necessarily undermine the oil’s efficacy, it highlights the need for careful dose optimisation to avoid potential adverse effects.
Table 1: Oral Acute Toxicity of Essential Oil from Azadirachta indica Leaves and Picralima nitida Seeds

Group A: Infected and treated with 300 mg/mL of Azadirachta indica oil
Group B: Infected and treated with 100 mg/mL of Azadirachta indica oil
Group C: Uninfected and treated with 300 mg/mL of Azadirachta indica oil
Standard Control: Infected and treated with 5 mg/mL of chloroquine
Table 2a: General Appearance and Behavioural Observations in Mice Administered with Azadirachta indica (A. Juss) Leaves during the Acute Toxicity Study

Table 2b: General Appearance and Behavioural Observations in Mice Administered with Picralima nitida Seeds during the Acute Toxicity Study

Table 3a: General Appearance and Behavioural Observations in Infected Mice Treated with Essential Oil from Azadirachta indica

Group A: Infected and treated with 300 mg/mL of Azadirachta indica oil
Group B: Infected and treated with 100 mg/mL of Azadirachta indica oil
Group C: Uninfected and treated with 300 mg/mL of Azadirachta indica oil
Standard Control: Infected and treated with 5 mg/mL of chloroquine
Table 3b: General Appearance and Behavioural Observations in Infected Mice Treated with Essential Oil from Picralima nitida
Group A: Infected and treated with 300 mg/mL of Picralima nitida (abere) oil
Group B: Infected and treated with 100 mg/mL of Picralima nitida (abere) oil
Group C: Uninfected and treated with 300 mg/mL of Picralima nitida (abere) oil
Standard Control: Infected and treated with 5 mg/mL of chloroquine
3.4 Comparison of Body Weight and Body Temperature in Mice Infected with P. berghei (Nk-65) During Treatment with Essential Oil from Picralima nitida and Azadirachta indica
The body weight of the group treated with 300 mg/mL and 100 mg/mL of the Picralima nitida essential oil remained unchanged at 17 g and 16 g, respectively, while those treated with the antimalarial drug showed a slight reduction from 17 g to 16.5 g. There was no significant change in the body weight of the mice treated with Azadirachta indica, with the exception of the group that was uninfected and treated with a high dosage of the essential oil, which showed a drastic reduction in average weight from 21 g to 15.6 g (see Figure 1).
The temperature of the group treated with 300 mg/mL of the Picralima nitida essential oil remained unchanged (36.7°C), while the group treated with 100 mg/mL of the Picralima nitida essential oil slightly reduced from 36.3°C to 36.1°C, and the group treated with the antimalarial drug (standard control) showed a decrease in temperature from 37.2°C to 36.2°C. There was a decrease in temperature in all experimental groups, with the exception of the group of mice treated with a high dosage of the essential oil from Azadirachta indica leaves, which showed a slight increase in temperature from 36.7°C to 36.9°C. The standard control mice group treated with chloroquine showed a decrease in temperature from 37.7°C to 36.2°C (see Figure 2).
3.5 Comparison of the Parasitaemia Load in Mice Infected with P. berghei (Nk-65) During Treatment with Essential Oil from Picralima nitida
The parasitaemia load of mice during and after the infection period is presented in Table 4. The parasitaemia load of the infected mice treated with 300 mg/kg of Azadirachta indica and Picralima nitida oil was 0% parasitaemia with 100% chemo-suppression after a 72-hour treatment period; the same scenario was observed in the chloroquine-treated group.

Figure 1: Changes in Weight of Infected Mice during Treatment
KEY: Group A -Infected and treated with oil 300 mg/ml of Azadirachta indic;, Group B- Infected and treated with oil 100 mg/ml of Azadirachta indica; Group C -Infected and treated with oil 300 mg/ml of Picralima nitida (abere); Group D- Infected and treated with oil 100 mg/ml of Picralima nitida (abere); Group E- Uninfected and treated with oil Azadirachta indica; Group F -Uninfected and treated with oil Picralima nitida (abere); Group G- Infected and Untreated; Group H – Infected and treated with anti-malarial drug; Group I= Uninfected and Untreated
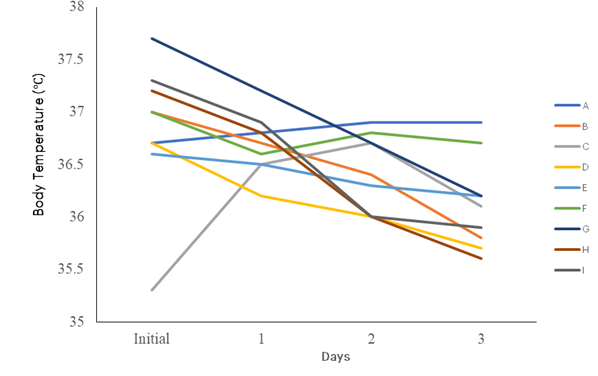
Figure 2: Changes in Temperature of Infected Mice during Treatment
KEYS: Group A -Infected and treated with oil 300 mg/ml of Azadirachta indica, Group B- Infected and treated with oil 100 mg/ml of Azadirachta indica, Group C -Infected and treated with oil 300 mg/ml of Picralima nitida (abere), Group D- Infected and treated with oil 100 mg/ml of Picralima nitida (abere),Group E- Uninfected and treated with oil Azadirachta indica, Group F -Uninfected and treated with oil Picralima nitida (abere), Group G- Infected and Untreated, Group H – Infected and treated with anti-malarial drug, Group I= Uninfected and Untreated
Table 4: Parasitaemia Load of Mice Infected with Plasmodium berghei (NK-65) During Treatment
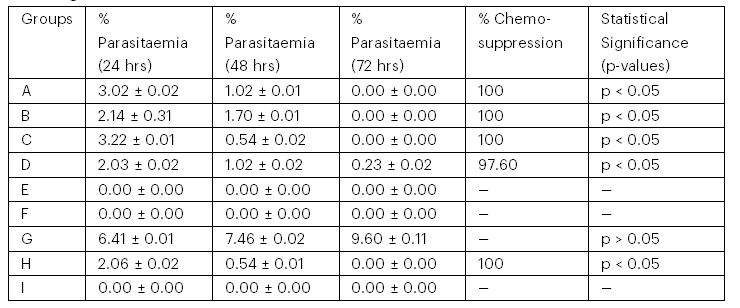
Keys:
Group A – Infected and treated with oil 300 mg/mL of Azadirachta indica
Group B – Infected and treated with oil 100 mg/mL of Azadirachta indica
Group C – Infected and treated with oil 300 mg/mL of Picralima nitida (abere)
Group D – Infected and treated with oil 100 mg/mL of Picralima nitida (abere)
Group E – Uninfected and treated with oil of Azadirachta indica
Group F – Uninfected and treated with oil of Picralima nitida (abere)
Group G – Infected and untreated
Group H – Infected and treated with an antimalarial drug
Group I – Uninfected and untreated
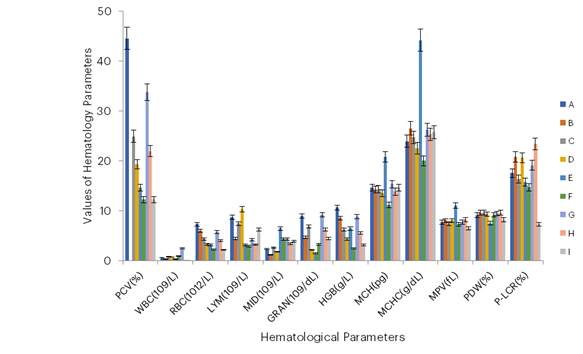
Figure 3: Effects of Essential Oil of Azadirachta indica Leaf and Picralima nitida Seeds
KEYS:
Group A -Infected and treated with oil 300 mg/ml of Azadirachta indica,
Group B- Infected and treated with oil 100 mg/ml of Azadirachta indica,
Group C -Infected and treated with oil 300 mg/ml of Picralima nitida (abere),
Group D- Infected and treated with oil 100 mg/ml of Picralima nitida (abere),
Group E- Uninfected and treated with oil Azadirachta indica,
Group F -Uninfected and treated with oil Picralima nitida (abere),
Group G- Infected and Untreated,
Group H – Infected and treated with anti-malarial drug,
Group I= Uninfected and Untreated
3.6 Effects of Oil on Liver Function Enzymes
The effects of oil on liver function enzymes in P. berghei-infected mice treated with oil from Azadirachta indica leaves and Picralima nitida seeds are shown in Figures 4 and 5, respectively.
3.6. 1 Aspartate Transaminase (AST)
The effects of essential oil from Azadirachta indica leaves on aspartate transaminase (AST) activity were evaluated in the serum of Plasmodium berghei-infected mice. At dosages of 300 mg/mL and 100 mg/mL, AST activity was higher, with values of 31 U/L and 47 U/L, respectively. These values were higher than in the standard control group, which had an AST value of 17.2 U/L. However, the AST activity in the treated groups was not significantly different from the untreated control group, which had a value of 33.2 U/L. In mice treated with Picralima nitida essential oil at a dosage of 300 mg/mL, the AST value was 65.8 U/L. In comparison, the negative control group had a value of 33.2 U/L, and the standard control group had a value of 17.2 U/L.
Elevated AST levels often indicate liver damage or dysfunction. The higher AST values in the experimental groups treated with Azadirachta indica or Picralima nitida essential oils could indicate that these treatments may have an impact on liver function. Elevated AST in the Picralima nitida-treated group (65.8 U/L) may indicate potential liver stress or toxicity.
3.6.2 Alanine Transaminase (ALT)
The impact of Azadirachta indica leaf essential oil on alanine transaminase (ALT) activity in the serum of Plasmodium berghei-infected mice was evaluated. ALT activity was normal in the standard control group (17.6 U/L), at a dosage of 300 mg/mL (20 U/L), and in the negative control group (16.3 U/L), but slightly higher at a dosage of 100 mg/mL (23 U/L). In the mice treated with Picralima nitida at a dosage of 300 mg/mL of the essential oil, an ALT value of 25.6 U/L was observed, compared with 17 U/L for the standard control group. In healthy mice, ALT levels typically fall within a certain range, and a value of 17 U/L might be considered normal. If the increase to 25.6 U/L is within the range expected for minor stress or variation, it may not represent significant biological damage.
3.6.3 Alkaline Phosphatase (ALP)
The effects of essential oil from Azadirachta indica leaves on alkaline phosphatase activity in the serum of Plasmodium berghei-infected mice were evaluated. ALP activity was normal across all experimental groups, with the following values: 25.7 U/L in the standard control group, 20.2 U/L in the negative control group, 17.2 U/L at 100 mg/mL, and 21 U/L at 300 mg/mL. In mice treated with Picralima nitida at a dosage of 300 mg/mL of the essential oil, an ALP value of 25.3 U/L was observed, with 20.2 U/L for the negative control group, and 25.7 U/L for the standard control group.
3.6.4 Total Proteins
Total protein values in Azadirachta indica-treated groups were normal at a dosage of 300 mg/mL (81 µg/µL) and in the standard control group (76.3 µg/µL). However, total protein levels were higher at a dosage of 100 mg/mL (87 µg/µL) and in the negative control group (94 µg/µL). In mice treated with Picralima nitida at a dosage of 300 mg/mL of the essential oil, a total protein value of 90.2 µg/µL was observed, compared with 96.3 µg/µL for the negative control group and 94.3 µg/µL for the standard control group.
3.6.5 Total Bilirubin
Total bilirubin values in A. indica-treated groups were normal in all experimental groups, with the following values: standard control group (18.3 µmol/L), 11 µmol/L and 7.4 µmol/L at 100 mg/mL and 300 mg/mL, respectively, and 16.3 µmol/L in the negative control group. In mice treated with Picralima nitida at a dosage of 300 mg/mL of the essential oil, 8.4 µmol/L of total bilirubin was observed, compared with 15.1 µmol/L for the negative control group and 16.3 µmol/L for the standard control group.
3.6.6 Albumin
The albumin value in A. indica-treated groups was normal in the standard control group (36.1 g/L), indicating an absence of hepatic stress. It was high at a dosage of 300 mg/mL, which may indicate an adaptive response, potentially related to compensatory mechanisms, increased protein synthesis, or dehydration, and was high in the negative control group with values of 57 g/L and 54.2 g/L, respectively, but low at a dosage of 100 mg/mL (33 g/L), which is indicative of hepatic stress or impaired liver function. In mice treated with Picralima nitida at a dosage of 300 mg/mL of the essential oil, an albumin value of 44.1 g/L was observed, with 54.2 g/L for the negative control group and 36.1 g/L for the standard control group.

Figure 4: Liver Function Enzymes of Infected Mice Treated with Azadirachta indica Oil
Key: Group A: infected and treated with 300 mg/ml of Azadirachta indica oil; Group B: infected and treated with 100 mg/ml of Azadirachta indica oil; Group C: uninfected and treated with 300 mg/ml of Azadirachta indica oil;Group D: infected and treated with Choroquine (5 mg/ml, standard control); Group E: uninfected and untreated (positive control); Group F: infected and untreated (negative control).

Figure 5: Liver Function Enzymes of Infected Mice Treated with Picralima nitida Oil
Key: Group A: infected and treated with 300 mg/ml of Picralima nitida (abere) oil; Group B: infected and treated with 100 mg/ml of Picralima nitida (abere) oil; Group C: uninfected and treated with oil Picralima nitida (abere); Group D: infected and untreated (negative control); Group E: infected and treated with Choroquine (5 mg/ml, standard control); Group F: uninfected and untreated (positive control).
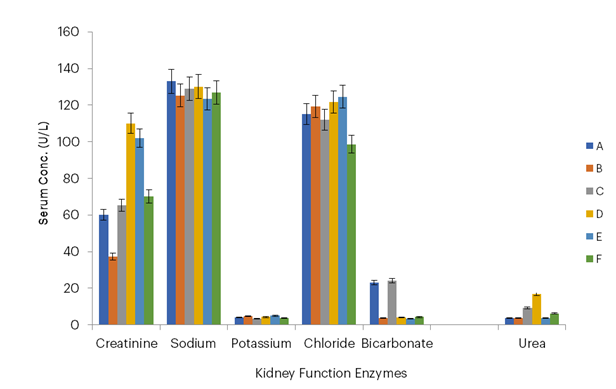
Figure 6: Kidney Function Enzymes of Infected Mice Treated with Azadirachta indica Oil
Key: Group A – Infected and treated with 300 mg/mL of Azadirachta indica oil; Group B – Infected and treated with 100 mg/mL of Azadirachta indica oil; Group C – Uninfected and treated with 300 mg/mL of Azadirachta indica oil; Group D – Infected and treated with chloroquine (5 mg/mL, standard control); Group E – Uninfected and untreated (positive control); Group F – Infected and untreated (negative control).
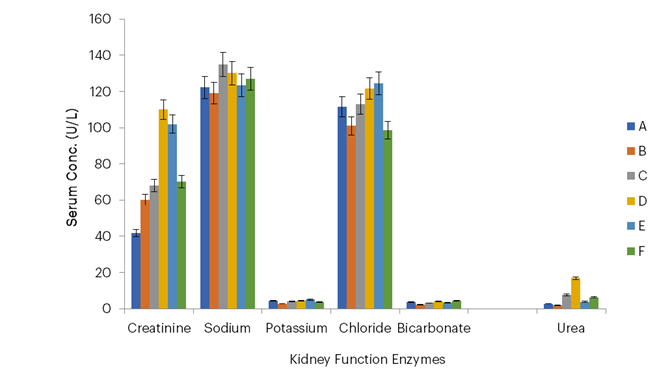
Figure 7: Kidney Function Enzymes of Infected Mice Treated with Picralima nitida Oil
Key: Group A – Infected and treated with 300 mg/mL of Picralima nitida (abere) oil; Group B – Infected and treated with 100 mg/mL of Picralima nitida (abere) oil; Group C – Uninfected and treated with oil of Picralima nitida (abere); Group D – Infected and untreated (negative control); Group E – Infected and treated with chloroquine (5 mg/mL, standard control); Group F – Uninfected and untreated (positive control).
4. Discussion
Over the past decades, various components of the A. indica (A. Juss) tree and P. nitida seeds have been employed in the treatment of diseases such as malaria, diabetes, gastrointestinal infections, and skin conditions due to their broad spectrum of biologically active compounds (Odukoya et al., 2021; Guo et al., 2024). This study, however, demonstrates the effects of essential oils from Azadirachta indica leaves and Picralima nitida seeds on the amelioration of renal and hepatic functions in mice infected with Plasmodium berghei (NK-65).
The use of experimental animal models for a disease not only enhances comprehension of its pathophysiology but also plays a crucial role in identifying and evaluating novel therapeutic interventions, as highlighted in this study, which explores the efficacy of A. indica as a potential alternative to synthetic antimalarial drugs (Guo et al., 2024).
The oral acute toxicity study showed that the LD₅₀ of the essential oil from Azadirachta indica leaves and Picralima nitida seeds was greater than 5000 mg/kg, indicating a wide safety margin. For comparison, common antimalarial agents such as chloroquine have reported LD₅₀ values in mice ranging from 150 to 330 mg/kg, highlighting the significantly higher safety threshold of these essential oils (USCDC, 2024). This comparison underscores the potential of these natural compounds as safer alternatives in antimalarial therapy.
This finding aligns with studies by Aina et al. (2022), who conducted acute toxicity assessments of aqueous and ethanolic extracts of P. nitida and found the LD₅₀ to be ≥2000 mg/kg. Additionally, no histopathological damage was observed in subjects administered 300 mg/kg, 2000 mg/kg, or 5000 mg/kg of the aqueous extract. Braga et al. (2021) orally administered different doses of aqueous extracts of A. indica leaves to mice (1250, 2500, and 5000 mg/kg b.w.) with no sign of toxicity.
There were no significant changes in behavioural patterns, body weight, or the relative weight of vital organs. Also, no mortality was recorded. No obvious toxic side-effects were observed and the treated mice were found to be healthy and normal, with no records of weight or hair loss, allergy, or other symptoms of discomfort. Thus, the LD₅₀ value was considered higher than 5000 mg/kg. An initial change in body temperature was, however, observed; this could be due to an initial disruption in the homeostatic balance of the mice’s internal environment.
The essential oils from A. indica showed 0% parasitaemia after a 72-hour treatment period, demonstrating complete clearance of the parasite. This is consistent with findings from previous studies that reported the high efficacy of various parts of the A. indica tree, including its essential oil, in malaria treatment (Igwenyi et al., 2024). In this study, 45 mice were treated, and the results were statistically significant (p < .05), with a 95% confidence interval indicating a high level of reliability.
The mice groups treated with essential oil from P. nitida seeds showed a significant reduction in parasitaemia load compared to the control group. This may be because the plant possesses strong antioxidant properties. Antioxidants help to neutralise harmful free radicals in the body, which can cause damage to cells and tissues. By reducing oxidative stress, P. nitida may indirectly increase antiplasmodial activity by enhancing the immune system’s ability to fight off the malaria parasite. A study carried out by Ogbuehi et al. (2018) similarly reported a dose-dependent reduction in parasitaemia load in mice treated with essential oil from P. nitida.
Anemia, a common symptom of malaria as noted by Kaur and Juneja (2022), was evident in the untreated control group in this study. However, treatment with A. indica and P. nitida essential oils significantly improved haematological parameters, suggesting their potential to ameliorate malaria-induced anaemia. Anaemia is normally evaluated by testing for red blood cell (RBC) count, packed cell volume (PCV) or haematocrit (HCT), haemoglobin (HGB), mean cell volume (MCV), mean cell haemoglobin (MCH), and mean cell haemoglobin concentration (MCHC). The study observed high haemoglobin, WBC, and PCV in the infected groups; this may be due to the destruction of infected red blood cells and the subsequent release of haemoglobin. The results of the PCV and RBC count in the animal model suggest that the essential oil extract has the potential to reverse anaemia, as evidenced by increased blood formation.
In this study, the PCV was observed to be normal in all groups infected and treated with the essential oil from A. indica leaves and P. nitida seeds at dosages of 100 mg/mL and 300 mg/mL, as well as in the standard control group treated with chloroquine. This indicates that the essential oils did not result in anaemia in the mice. This agrees with the findings of Igwenyi et al. (2024), who found that A. indica fruit juice replenished blood levels in P. berghei-infected mice.
However, the WBC count was low in all groups. This might suggest that the influx of blood cells from the bone marrow did not align with their removal rate from the circulation. Alternatively, it could be attributed to a deficiency in the production of haematopoietic regulatory elements by the stromal cells and macrophages in the bone marrow at the given doses. This is in line with the findings of Alaa et al. (2022), who carried out a study on the toxicity of A. indica oil.
The essential oil at all doses increased AST activity. Elevated AST levels observed in the bloodstream of untreated, parasite-infected mice likely result from liver damage and structural changes in hepatocytes caused by Plasmodium infection, leading to the release of these enzymes into the bloodstream. In comparison, normal AST levels in healthy mice typically range around 17 U/L, highlighting the significant deviation observed in the infected, untreated groups. This result is in agreement with the findings of Kouam et al. (2023). ALT activity, as well as total proteins, were high at a dosage of 100 mg/mL, which falls within the physiological range. This is consistent with other studies that reported that the majority of malaria patients show elevated serum enzyme activities indicating liver damage (Megabiaw et al., 2024). ALT activity and total protein levels were, however, normal at a dosage of 300 mg/mL for A. indica and 100 mg/mL for P. nitida, which suggests that the oils work best at high concentrations and low concentrations, respectively.
However, albumin was high at a dosage of 300 mg/mL as well as in the negative control group, and low at a dosage of 100 mg/mL. This indicates the onset of liver damage, which also agrees with the findings of Megabiaw et al. (2024). Albumin levels were, however, normal at both dosages (100 mg/mL and 300 mg/mL) for the mice groups treated with essential oil from P. nitida seeds, which suggests that the oil was able to protect hepatocyte integrity. It also indicates that the essential oil from A. indica leaves selectively affected some liver enzymes while others were unaffected (El-Zaiat et al., 2022).
In this study, the lowest urea concentrations were observed at dosages of 100 mg/mL and 300 mg/mL for P. nitida, with values of 1.9 g/dL and 2.7 g/dL, and 3.6 g/dL and 3.7 g/dL, respectively, for A. indica, compared with the negative control group which had a value of 6.2 g/dL. Reduced urea levels may indicate improved kidney function, as the kidneys efficiently filter and excrete urea, a waste product of protein metabolism. These findings suggest that P. nitida may enhance renal clearance or reduce the production of urea through its potential anti-inflammatory or antioxidant effects, thereby mitigating renal stress. This aligns with the hypothesis that the essential oil extracts may play a protective role in maintaining or restoring normal kidney function in Plasmodium-infected mice.
Also, the lowest creatinine concentrations were observed at dosages of 100 mg/mL and 300 mg/mL with values of 60.2 g/dL and 40.1 g/dL, respectively, for P. nitida, and 37.2 g/dL and 60.1 g/dL, respectively, for A. indica, compared with the negative control group which had a value of 70.1 g/dL. Maintenance of kidney integrity by medicinal plants was reported by Mohammed et al. (2021).
The blood electrolyte levels—sodium, potassium, chloride, and bicarbonate—in mice treated with essential oils from A. indica leaves and P. nitida seeds were comparable to those in the standard control group treated with chloroquine. This finding underscores the potential efficacy of these essential oils as antimalarial agents, as they not only combat the parasitic infection but also maintain electrolyte balance, a critical indicator of systemic stability and kidney function. The comparable results with chloroquine highlight their promise as alternative, natural treatments for malaria. This shows that the essential oils were as effective as chloroquine in the amelioration of renal and hepatic functions in the malaria-infected mice.
6. Conclusion
The acute toxicity study of essential oils from A. indica leaves and P. nitida seeds in mice indicated no signs of toxicity, which shows that the essential oils have a good safety profile. This study also demonstrates that the essential oils have promising antiplasmodial activity, with no side effects or risk of anaemia. The essential oils have the potential to ameliorate renal and hepatic functions, reducing the extent of damage caused by malaria. These findings suggest that the essential oils from A. indica leaves and P. nitida seeds could serve as potential candidates for further development as alternatives to synthetic drugs in the treatment of malaria.
7. Recommendations
Based on this study, we recommend that the essential oils from Azadirachta indica leaves and Picralima nitida seeds be considered as prospective alternatives to synthetic drugs such as chloroquine in the treatment of malaria, as they have shown promising capabilities for the amelioration of renal and hepatic functions, have been proven to be safe, and may contribute towards overcoming the challenge of antimicrobial resistance (AMR) associated with existing antimalarial drugs. Additionally, further toxicological investigations, including sub-acute, sub-chronic, and chronic toxicity assessments, are imperative to evaluate the enduring impacts of the essential oils.
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this research.
References
Aina, O., Okoyenta, O., Kareem, K., Bamgbose, D., Olusola, A., & Afolabi, B. (2022). Toxicological studies of Picralima nitida aqueous and ethanolic extracts in an animal model. Toxicological Studies of Picralima Nitida, 21(1), 21–30.
Alaa, E. B., Abdel, R. H., Nadia, Z. D., & Dalia, A. Y. (2022). Toxicity evaluation of two Neem oil nano formulations using Swiss male albino mice. Biomedical Journal of Science & Technical Research, 43, 2574–1241.
Braga, T. M., Rocha, L., Chung, T. Y., Oliveira, R. F., Pinho, C., Oliveira, A. I., Morgado, J., & Cruz, A. (2021). Azadirachta indica A. Juss. In vivo toxicity—An updated review. Molecules, 26(2), 252.
Dong, A., Dong, H., Zhang, T., Jing, X., He, H., & Huo, J. (2024). The acute toxicity of cadmium on turtle Mauremys reevesii. Aquatic Ecology, 58(4), 1217–1223.
El-Zaiat, H. M., Elshafie, E. I., Al‑Marzooqi, W., & Dughaishi, K. A. (2022). Effects of Neem (Azadirachta indica) leaf powder supplementation on rumen fermentation, feed intake, apparent digestibility and performance in Omani sheep. Animals, 12, 3146.
Evbuomwan, I. O., Adeyemi, O. S., & Oluba, O. M. (2023). Indigenous medicinal plants used in folk medicine for malaria treatment in Kwara State, Nigeria: An ethnobotanical study. BMC Complementary Medicine and Therapies, 23(1), Article 324.
Faisal, U. M., Saifi, M. S., Kaish, M., Ibrahim, M., Kwakuri, S. S., & Arif, M. (2023). Azadirachta indica (Neem): An important medicinal plant: A literature review of its chemistry, biological activities, role in COVID-19 management and economic importance. Journal of Pharmacognosy and Phytochemistry, 12(6), 59–65.
Figueroa-Romero, A., Pons-Duran, C., & Gonzalez, R. (2022). Drugs for intermittent preventive treatment of malaria in pregnancy: Current knowledge and way forward. Tropical Medicine and Infectious Disease, 7(8), 152.
Frischknecht, F. (2023). Life cycle of malaria-causing parasites. In Malaria: Deadly parasites, exciting research and no vaccination (pp. 9–18). Wiesbaden: Springer Fachmedien Wiesbaden.
Guo, H., Xu, X., Zhang, J., Du, Y., Yang, X., He, Z., … & Guo, L. (2024). The pivotal role of preclinical animal models in anti‑cancer drug discovery and personalised cancer therapy strategies. Pharmaceuticals, 17(8), 1048.
Hanboonkunupakarn, B., Tarning, J., Pukrittayakamee, S., & Chotivanich, K. (2022). Artemisinin resistance and malaria elimination: Where are we now? Frontiers in Pharmacology, 13, 876282.
Igwenyi, I. O., Onodugo, C. A., Aja, P. M., Elom, S. O., Awoke, J. N., Ibhadode, O. S., … & Atoki, A. V. (2024). Azadirachta indica fruit juice clears malaria parasites and replenishes blood levels in Plasmodium berghei-infected mice. Phytomedicine Plus, 4(4), 100615.20.
Johnson, J. T., & Modo, E. U. (2024). Effect of Liv. 52hb, Ganoderma lucidum, Stc30, and astaxanthin on serum TNF‑α and liver function indices following CCl₄‑induced hepatocellular carcinoma in Albino Wistar rat model. International Journal of Research in Medical and Clinical Science, 2(1), 25–36.
Kaur, M., & Juneja, R. (2022). An analysis of the relation between malaria and anaemia. International Journal of Innovative Research in Engineering & Management, 9(1), 303–306.
Kebede, F. (2023). Status, drivers, and suggested management scenarios of salt‑affected soils in Africa. In Biosaline Agriculture as a Climate Change Adaptation for Food Security (pp. 259–284). Cham: Springer International Publishing.
Kouam, A. F., Ngoumé, N. A. N., Fepa, A. G. K., Wainfen, Z., Ngounou, E., Galani, B. R. T., Nembo, N. E., Chuisseu, P. D. D., Njayou, F. N., & Moundipa, P. F. (2023). Liver injury in malaria‑infected patients in Douala‑Cameroon and its association with poor medical practice. Egyptian Liver Journal, 13, 67.
Martins, R. X., Carvalho, M., Maia, M. E., Flor, B., Souza, T., Rocha, T. L., & Farias, D. (2024). 2,4‑D herbicide‑induced hepatotoxicity: Unveiling disrupted liver functions and associated biomarkers. Toxics, 12(1), 35.
Megabiaw, F., Eshetu, T., Kassahun, Z., & Aemero, M. (2022). Liver enzymes and lipid profile of malaria patients before and after antimalarial drug treatment at Dembia Primary Hospital and Teda Health Center, Northwest Ethiopia. Research and Reports in Tropical Medicine, 13, 11–23.
Mehlhorn, H. (2023). Protozoans attacking humans. In Human Parasites: Diagnosis, Treatment, Prevention (pp. 19–129). Cham: Springer International Publishing.
Mohammed, R. R., Omer, A. K., Yener, Z., Uyar, A., & Ahmed, A. K. (2021). Biomedical effects of Laurus nobilis L. leaf extract on vital organs in streptozotocin‑induced diabetic rats: Experimental research. Annals of Medicine and Surgery, 61, 188–197.
Muganda, A. (2022). Outcome of chronic schistosomiasis in the regulation of malaria disease severity and pathological events in a mouse model (Doctoral dissertation, Technical University of Kenya).
Munir, A. (2024). Investigating drug repositioning as a route to combat drug resistance in Plasmodium falciparum malaria (Doctoral dissertation). University of Oxford.
Odukoya, J. O., & Ndinteh, D. T. (2021). Elemental measurements and health risk assessment of sub‑Saharan African medicinal plants used for cardiovascular diseases’ and related risk factors’ treatment. Journal of Trace Elements in Medicine and Biology, 65, 126725.
Ogbuehi, I. H., Nwinyi, F., & Osadebe, P. O. (2018). Antimalarial activity of Picralima nitida in a model of malaria. Parasitology Research, 117(2), 449–456.
Okagu, I. U., Aguchem, R. N., Ezema, C. A., Ezeorba, T. P. C., Eje, O. E., & Ndefo, J. C. (2022). Molecular mechanisms of haematological and biochemical alterations in malaria: A review. Molecular and Biochemical Parasitology, 247, 111446.
Rosado-Quiñones, A. M., Colón-Lorenzo, E. E., Pala, Z. R., Bosch, J., Kudyba, K., Kudyba, H., … & Serrano, A. E. (2024). Novel hydrazone compounds with broad‑spectrum antiplasmodial activity and synergistic interactions with antimalarial drugs. Antimicrobial Agents and Chemotherapy, 68(6), e01643‑23.
Shaheen, S., & Sarwar, S. (2024). Herbal wisdom through time: The evolution of medicinal plants utilisation. In Plants as Medicine and Aromatics (pp. 343–356). CRC Press.
United States Centre for Disease Control. (2024). Malaria – Drug resistance in the malaria‑endemic world.
World Health Organization. (2024a). Fourth annual global forum of malaria‑eliminating countries: Meeting report, Cape Town, South Africa, 24–26 January 2023. World Health Organization.
World Health Organization. (2024b). Global Malaria Programme operational strategy 2024–2030. World Health Organization.
Yusuf, H. U., & Aliyu-Paiko, M. (2020). Evaluation of cashew nut meal as phytobiotics in the diet of broiler chickens and effects on feed efficiency, growth performance, blood metabolic and antioxidant profiles. International Journal of Veterinary Sciences and Animal Husbandry, 5(1), 108–114.
Zimmermann, M. (1983). Ethical guidelines for the investigation of experimental pain in conscious animals. Pain, 16(2), 109–110.
About this Article
Cite this Article
APA
Omoya F. O., Ajayi K. O., Fasanya K., Oladele O., Famuyiwa V., & Ejuwa K. (2025). Comparative Studies on Amelioration of Renal and Hepatic Functions by Essential Oil of Picralima nitida Seeds and Azadirachta indica Leaves in Mice Infected by Plasmodium berghei (NK-65). In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Omoya F. O., Ajayi K. O., Fasanya K., Oladele O., Famuyiwa V., and Ejuwa K. 2025. “Comparative Studies on Amelioration of Renal and Hepatic Functions by Essential Oil of Picralima nitida Seeds and Azadirachta indica Leaves in Mice Infected by Plasmodium berghei (NK-65).” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
30 October 2024
Accepted
7 January 2025
Published
4 February 2025
Corresponding Author Email: ajayikehinde@pcu.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














