Agboola, S. A. 1 Olaniyi O. O. 1 Adeleke, B. S. 1 Akinyele, J. B. 1 Amos-Agboola, O. Y. 1
1Department of Microbiology, Federal University Oye-Ekiti, Ekiti-State, Nigeria
*Corresponding Author Email:saagboola@futa.edu.ng
Abstract
Recently, African researchers have been looking for ways to improve the nutritional components of some fermented indigenous foods using natural materials that are environmentally friendly. This study aimed to investigate the nutritional and antioxidant properties of fermented kunu using Lactobacillus plantarum, Lb. fermentum, Bacillus subtilis and Saccharomyces cerevisiae isolates. Isolation of microorganisms from the kunu samples using serial dilution and pour plate techniques was conducted. Fermentation of kunu using Lb. plantarum, Lb. fermentum, B. subtilis and S. cerevisiae isolates was evaluated using standard inoculation methods. The nutritional and antioxidant properties of the kunu were then determined. The microorganisms isolated from the kunu samples included Serratia marcescens, Staphylococcus aureus, Bacillus subtilis, Lactobacillus plantarum, Lb. fermentum, Aspergillus niger, A. flavus, Escherichia coli and Saccharomyces cerevisiae. The inoculants used were Lb. plantarum, Lb. fermentum, S. cerevisiae and B. subtilis. The protein (4.01%), ash (2.54%), fibre (6.98%) and moisture contents (85.01%) of kunu from mono- and co-cultural fermentation increased significantly, while fat (1.14%) and carbohydrate (6.70%) decreased when compared with naturally fermented kunu. The total phenolic (3.34 mg/g) and flavonoid (1.40 mg/g) contents of kunu from mono- and co-cultural fermentation increased compared with naturally fermented kunu. Therefore, the improvement in the nutritional content of the fermented kunu sample with synergistic action of the microorganisms suggests that it can be a good source of protein and probiotics.
Keywords: Nutrition, Antioxidant, Protein, Fermentation, Inoculation.
1. Introduction
Cereals such as millet, maize (Zea mays), sorghum (Sorghum bicolor) and barley (Hordeum vulgare) form major food components in various traditional diets, especially among the less affluent individuals in society (Amit & Yashbir, 2024). Consumption of millet and millet products confers considerable therapeutic and nutritional benefits (Gahalawat et al., 2024).
Local fermentation technology is employed in the processing of millet into desirable products with high nutritional contents (Ajagekar et al., 2023). Fermentation is the main precursor in the production of different fermented products, enhancing the nutritional composition of foods through the biosynthesis of vitamins, essential amino acids and proteins, improved bioavailability of micronutrients, and the degradation of antinutritional factors (Senanayake et al., 2023). It has been reported that fermentation promotes food safety, reduces food toxicity and aids in the production of antimicrobial agents (Sharma et al., 2020).
Millet can be processed into various forms such as ogi, fura, burukutu, bushera, koko, mangisi, jandh, togwa, ben-saalga, uji, dambu, roti, and kunu. Kunu is a non-alcoholic fermented beverage produced from millet. It is widely consumed in the northern parts of Nigeria, especially during the dry season (Olatoye et al., 2023). Kunu forms an essential component of the diet in many developing countries and is consumed either as a main dish or as a condiment (Ndukwe et al., 2023).
Kunu processing is commonly carried out by women using simple household equipment and utensils. The processing of kunu can be fortified using different spices such as ginger, clove, black pepper and ehuru (Adeniji & Ayoade, 2014). The microorganisms involved during kunu processing play active roles in the physical, nutritional and organoleptic modification of the substrates (Ndukwe et al., 2023). Mono- and co-cultural techniques have proven successful in enhancing the nutritional components of most fermented foods and in the synthesis of biomolecules (Omotade et al., 2024). Recently, little information has been available on the mono- and co-cultural potential of the microorganisms isolated from kunu.
Although fermentation of millet for kunu production using traditional processing methods involving natural microflora has proven successful in terms of nutritional quality, this study was designed to evaluate the nutritional and antioxidant composition of fermented kunu using different microorganisms isolated from kunu.
2. Material and Methods
2.1 Collection of Samples
Five different kunu samples (500 ml each) were purchased from different hawkers in various locations in Akure metropolis, namely Nepa Market, Oja-Oba Market, Isinkan Market, Ilesha Garage (Motor Park) and the Federal University of Technology, Akure Junior Staff Quarters. The samples were collected in sterile bottles. The raw millet (Pennisetum glaucum) (500 g) was also purchased at Isinkan Market, Akure, in sterile transparent polyethylene bags, well labelled, and immediately transported to the Microbiology Laboratory, Federal University of Technology, Akure for further analyses.
2.2 Preparation of the Kunu
This was carried out according to the method described by Abidoye (2017). Approximately 500 g of cleaned millet were weighed, steeped, washed and wet milled with the addition of spices (ginger, red pepper, black pepper and cloves). The wet-milled millet was sieved, and the supernatant was decanted to obtain a clear slurry. The slurry was divided into two unequal parts; two-thirds (75%) was added to boiling water, stirred and then cooled to 35 ± 2 °C; subsequently, the remaining one-quarter (25%) was added. The mixture was thoroughly stirred and sweetened with 10% granulated sugar. The product obtained (kunu) was divided into two portions; one portion was packed into sterile plastic bottles and pasteurised at 75 °C for 30 minutes and then allowed to cool, while the other portion (unpasteurised) served as the control.
2.3 Microbiological Analysis
Ten millilitres (10 ml) of each sample were homogenised in 90 ml of sterile peptone water to form the stock cultures. One millilitre (1.0 ml) from the stock culture was pipetted and serially diluted to appropriate dilutions of 10⁻⁵ and 10⁻³ for bacteria and fungi, respectively. Nutrient agar, de Man’s Rogosa Sharpe agar (MRSA), potato dextrose agar and yeast extract agar were prepared according to the manufacturers’ guidelines and used for culturing total bacterial, lactic acid bacterial, fungal and yeast isolates, respectively. Fungal plates were incubated at 28 °C for 72–96 hours, bacterial plates were incubated at 37 °C for 24 hours, and MRS agar plates were incubated anaerobically at 28 °C for 24–48 hours. Pure isolates were obtained by repeated streaking on freshly prepared microbiological media. Characterisation and identification of the isolated microorganisms were based on their cultural, microscopic, morphological and biochemical tests (Muthukumar & Dakshayini, 2021).
2.4 Inoculum Preparation
The inocula were prepared using 0.5 McFarland turbidity standards. The McFarland solution was prepared by adding 9.95 ml of 1% H₂SO₄ to 0.05 ml of 1% BaCl₂. The absorbance of the solution was then checked using a spectrophotometer at 623 nm. The microbial suspensions were prepared by adding a fresh culture of the isolates into sterile distilled water in a test tube until the turbidity matched the McFarland standard, representing 1.5 × 10⁸ cfu/ml for bacteria and 1.5 × 10⁸ sfu/ml for yeast suspension. Two hundred millilitres (200 ml) of pasteurised kunu was distributed into different fermenting vessels. Bacterial suspensions of 1.0 × 10³ cfu/ml and 1.0 × 10³ sfu/ml of yeast suspension each were used for mono- and co-cultural fermentation at ambient temperature (Eduardo et al., 2018).
2.5 Total Titratable Acidity and pH
The pH and titratable acidity of the kunu were monitored over 2 days. The percentage total titratable acidity was determined by diluting 10 ml of the sample in 90 ml of sterile distilled water. The mixture was homogenised and allowed to settle, from which 20 ml was titrated against 0.1 M NaOH using phenolphthalein as an indicator (Ire et al., 2020). The pH was determined using a pH meter (Crison Basic model 20) calibrated with standard buffers (pH 7.0 and 4.0).
2.6 Proximate Composition
The proximate composition of the fermenting kunu was determined according to AOAC (2012). The parameters measured included moisture, crude protein, crude fat, ash, fibre and carbohydrate contents.
2.7 Antioxidant Factors
The antioxidant properties of the kunu were determined as described by Zhishen et al. (1999), modified by Olatoye et al. (2023). The antioxidant factors measured were total phenolic and flavonoid contents.
2.8 Statistical Analysis
The data obtained were analysed and are presented as mean ± standard deviation. The significance of differences between groups was tested using one-way analysis of variance (ANOVA) with SPSS Windows Version 23 software. Differences were considered statistically significant at p < .05.
3. Results
3.1 Total Microbial Counts from Kunu Samples
Figure 1 shows the yeast’s initial ethanol tolerance before adaptation. After a 72-hour culture period on yeast extract potato dextrose agar and broth, the isolated wild S. cerevisiae showed visible growth at 0.5g/l, 1.0g/l, 1.5g/l… and 7.0g/l of ethanol. At 7.5g/l—11g/ll, there was no visible growth, indicating that at 7.5g/l, ethanol had a tidal effect on this wild strain of S. cerevisiae with tolerance of below 7.5g/l.
Table 1 shows the total microbial counts from kunu samples. The sample purchased from Nepa Market, Akure had the highest bacterial count (20.33 × 10⁵ cfu/ml on nutrient agar), followed by Isinkan Market, Akure (19.00 × 10⁵ cfu/ml), while the lowest bacterial count (3.00 × 10⁵ cfu/ml) was obtained from the prepared sample on MRS agar. Similarly, the highest fungal count (7.67 × 10³ sfu/ml) was obtained from the sample purchased from Ilesha Garage, Akure on yeast extract agar, followed by the sample from Nepa Market, Akure on potato dextrose agar, while the lowest fungal count (2.33 × 10³ sfu/ml) was obtained from the prepared sample on yeast extract agar.
Table 1: Total Microbial Counts Obtained from Kunu Samples

Data are represented as mean ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05). Key: NA = Nutrient agar; MRS = de Man’s Rogosa Sharpe agar; YEA = Yeast extract agar; PDA = Potato dextrose agar; JSQ = Junior Staff Quarters, FUTA; IMA = Isinkan Market, Akure; OMA = Oja-Oba Market, Akure; NMA = Nepa Market, Akure; IGA = Ilesha Garage, Akure; PKL = Prepared kunu in laboratory.
3.2 Colonial, Morphological and Biochemical Characterisation of Yeast and Bacterial Isolates
The colonial, morphological and biochemical characterisation of yeast and bacterial isolates from kunu samples is presented in Table 2.
Table 2: Colonial, Morphological and Biochemical Characterisation of Yeast and Bacterial Isolates from Kunu Samples
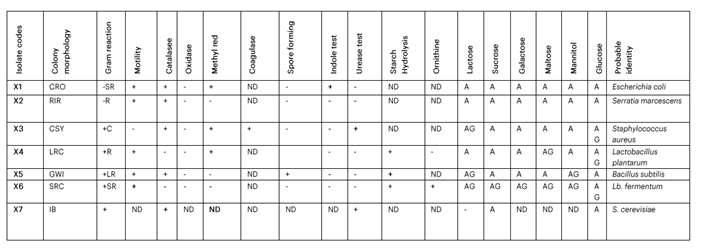
Keys: CRO = cream, opaque, round and flat elevation; RIR = red irregular, raised; CSY = circular, small, yellow; LRC = long rod, creamy; GWI = grey white, opaque irregular shape; SRC = short rod; R = rod; C = cocci; LR = long rod; ND = not determined; (–) = negative; (+) = positive; AG = acid production with gas; A = acid production; IB = internal budding.
3.3 Cultural Morphology and Microscopic Observation of Mold Isolated from Kunu Samples
The cultural morphology and microscopic observation of fungal isolates from kunu samples are presented in Table 3. The probable identities of the fungal isolates were Aspergillus flavus and A. niger
Table 3: Cultural Characterisation and Microscopic Observation of Mold Isolated from Kunu Samples
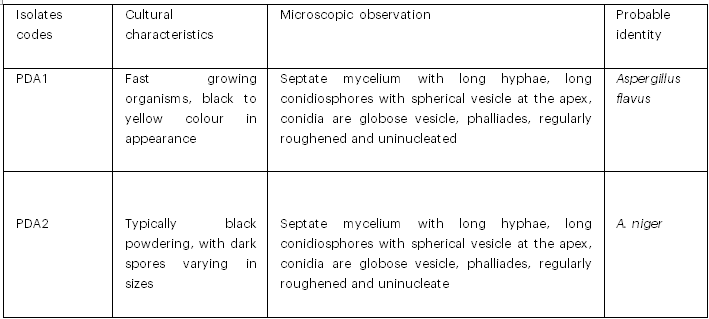
Key: PDA = Potato dextrose agar
3.4 pH of Kunu from Mono- and Co-Cultural Fermentation
The pH of kunu from mono- and co-cultural fermentation is represented in Table 4. The highest pH value (7.40) was obtained in the sample inoculated with B. subtilis, while the lowest pH (3.40) was obtained with the kunu sample inoculated with Lb. fermentum and Lb. plantarum at 48 hours of fermentation.
Table 4: pH of Kunu from Mono- and Co-cultural Fermentation
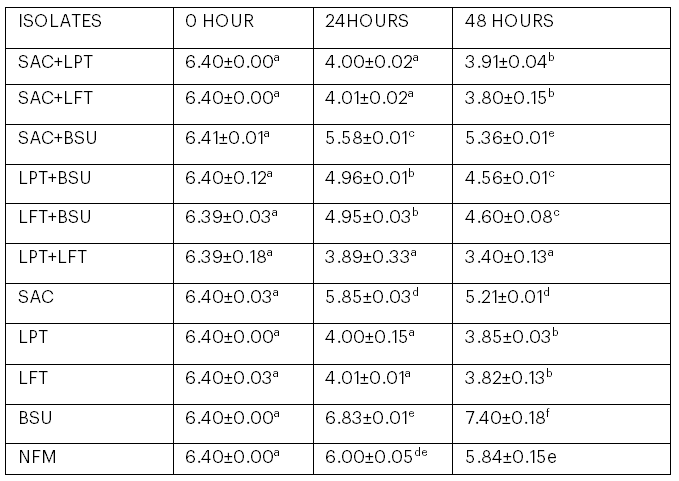
Values are means of triplicate determinations ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05).
Key: SAC = Saccharomyces cerevisiae; LPT = Lactobacillus plantarum; LFT = Lb. fermentum; BSU = Bacillus subtilis; SAC+LFT = S. cerevisiae and Lb. fermentum; SAC+LPT = S. cerevisiae and Lb. plantarum; SAC+BSU = S. cerevisiae and B. subtilis; LPT+LFT = Lb. fermentum and Lb. plantarum; LPT+BSU = Lb. plantarum and B. subtilis; LFT+BSU = Lb. fermentum and B. subtilis; NFM = Naturally fermented kunu.
3.5 Total Titratable Acidity of Kunu from Mono- and Co-cultural Fermentation
The total titratable acidity of kunu from mono- and co-cultural fermentation is represented in Table 5. The sample inoculated with Lb. plantarum and Lb. fermentum had the highest total titratable acidity (0.81) at 48 hours, while the sample inoculated with B. subtilis had the lowest value (0.13).
Table 5: Total Titratable Acidity of Kunu from Mono- and Co-cultural Fermentation
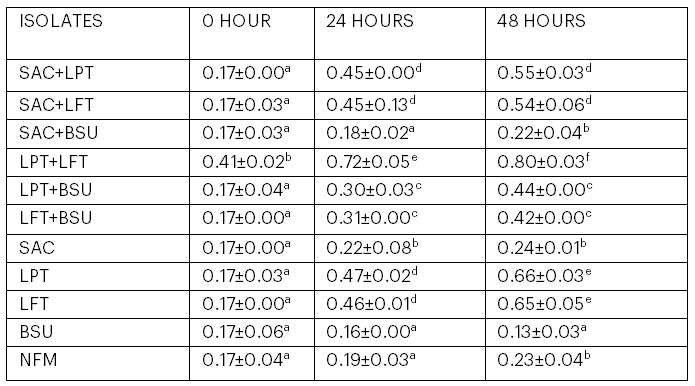
Values are means of triplicate determinations ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05).
Key: SAC = S. cerevisiae; LPT = Lb. plantarum; LFT = Lb. fermentum; BSU = B. subtilis; NFM = Naturally fermented kunu.
3.6 Proximate Composition of Kunu from Mono-Cultural Fermentation
The proximate composition of kunu from mono-cultural fermentation shows an increase in protein, ash, fibre and moisture content and a decrease in crude fat and carbohydrate content compared with naturally fermented kunu (Tables 6). Protein, ash, fibre and moisture contents increased from 1.37% to 2.98%, 0.46% to 1.70%, 6.32% to 6.98% and 84.01% to 85.01%, respectively, while crude fat and carbohydrate content decreased from 1.14% to 0.70% and 6.90% to 4.60%, respectively.
Table 6: Proximate Composition of Kunu from Mono-cultural Fermentation

Values are means of triplicate determinations ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05).
Key: SAC = S. cerevisiae; LPT = Lb. plantarum; LFT = Lb. fermentum; BSU = B. subtilis; NFM = Naturally fermented kunu.
3.7 Proximate Composition of Kunu from Co-cultural Fermentation
The proximate composition of kunu from co-cultural fermentation shows an increase in protein and ash content and a decrease in crude fat, fibre, moisture content and carbohydrate content compared with naturally fermented kunu (Tables 7). Protein and ash content increased from 1.37% to 4.00% and 0.46% to 2.50%, respectively, while crude fat, fibre, moisture content and carbohydrate content decreased from 1.14% to 0.62%, 6.32% to 4.34%, 84.01% to 72.81% and 6.70% to 3.99%, respectively.
Table 7: Proximate Composition of Kunu from Co-cultural Fermentation
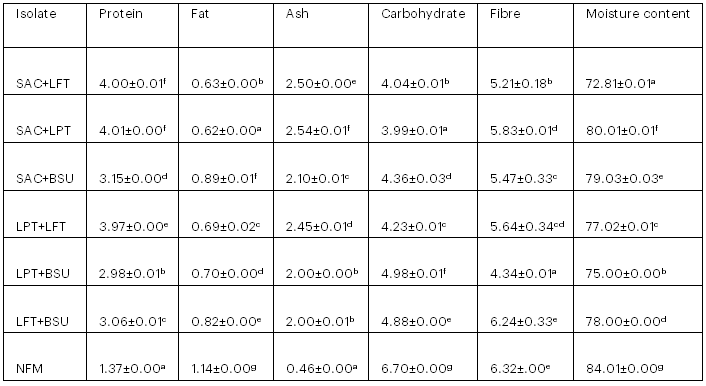
Values are means of triplicate determinations ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05).
Key: SAC+LFT = S. cerevisiae + Lb. fermentum; SAC+LPT = S. cerevisiae + Lb. plantarum; SAC+BSU = S. cerevisiae + B. subtilis; LPT+LFT = Lb. fermentum + Lb. plantarum; LPT+BSU = Lb. plantarum + B. subtilis; LFT+BSU = Lb. fermentum + B. subtilis; NFM = Naturally fermented kunu.
3.8 Antioxidant Profile of Kunu from Mono- And Co-Cultural Fermentation
The antioxidant profile of kunu from mono- and co-cultural fermentation is presented in Table 8. The results showed a considerable increase in the flavonoid and phenolic content of kunu from mono- and co-cultural fermentation compared with naturally fermented kunu. The highest flavonoid content of 1.40 mg/g and phenolic content of 3.34 mg/g were observed in the sample fermented with Lb. plantarum and Lb. fermentum, compared with naturally fermented kunu.
Table 8: Antioxidant Profile of Kunu from Mono- and Co-cultural Fermentation
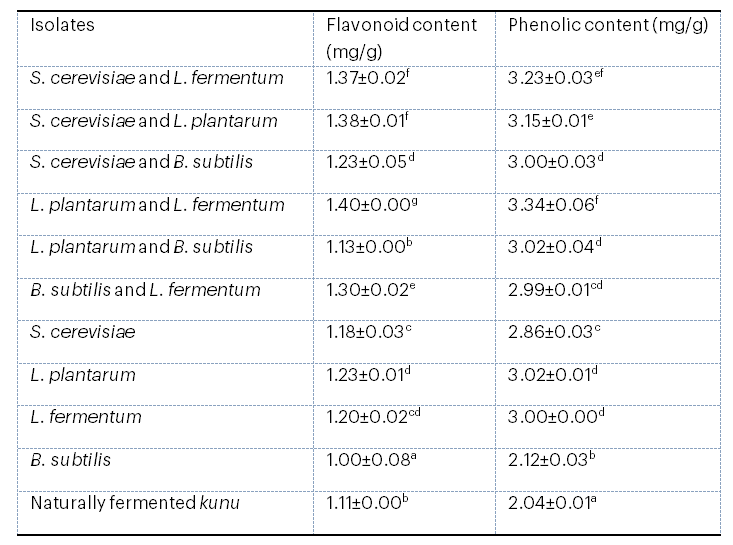
Values are means of triplicate determinations ± standard deviation. Means in the same column with different superscripts are significantly different (p < .05).
4. Discussion
Adequate utilisation of fermented foods depends on the availability of nutrients that meet consumer needs. Many fermented foods are well known but remain underutilised due to the presence of antinutritional factors and improper processing. Fermentation techniques contribute to improved nutritional profiles and reduced antinutritional contents of fermented foods (Anyiam et al., 2023). Various microorganisms associated with fermented foods significantly enhance their organoleptic properties. Several researchers have reported the isolation of diverse microorganisms from fermented cereal-based local beverages (Bouakkaz et al., 2024). The highest microbial counts obtained from Nepa Market, Akure, may be due to the processing methods used, contamination from handlers, processing utensils and exposure to air or dust at the point of sale (Erinle & Ajayi, 2022). Conversely, lower microbial counts may be attributed to better hygienic conditions and controlled processing environments. For example, the highest bacterial counts (8.00 cfu/ml) from kunu drinks sold in Port Harcourt metropolis have been reported (Essien et al., 2009). However, it is generally stated that the standard limit of <10⁵ cfu/ml is permissible for aerobic mesophilic bacteria in food (FDA, 2013).
The occurrence of Staphylococcus aureus, Serratia marcescens, E. coli, Aspergillus niger and A. flavus may be attributed to opportunistic contamination from air, human activity, or the natural flora of the grains used, as well as contamination from equipment (e.g. milling machines) employed in the preparation of kunu. The use of Lb. fermentum, Lb. plantarum, S. cerevisiae and B. subtilis in the fermentation process of kunu samples was based on reports that these microorganisms improve the nutritive value of some fermented foods (Bouakkaz et al., 2024). Lactic acid bacteria, notably Lactobacillus plantarum and Lb. fermentum, and yeast, which are typically present in fermented drinks or foods, serve as biopreservatives, thereby enhancing the organoleptic and nutritional properties of fermented foods (Wang et al., 2021). Zhina et al. (2021) suggested that yeast and bacteria exist in a symbiotic association, with the bacteria providing the acidic environment required for yeast growth, while the yeast supplies vitamins and other growth factors for the lactic acid bacteria.
The results of this study showed a decrease in pH and an increase in total titratable acidity of kunu from mono- and co-cultural fermentation compared with naturally fermented kunu. This increase in acidity and decrease in pH may be attributed to the abundant production of organic acids, such as lactic acid, by these microorganisms (Sionek et al., 2023). These findings agree with those of Nwachukwu et al. (2010), who reported a decrease in pH of akamu and kunu during fermentation using lactic acid bacteria.
The proximate composition of kunu from mono- and co-cultural fermentation revealed an increase in ash, fibre, crude protein and moisture content, and a decrease in carbohydrate and fat content compared with naturally fermented kunu. The increase in crude protein content in inoculated kunu may be due to the high number of microorganisms capable of secreting extracellular proteolytic enzymes into the fermenting medium (Jabbar et al., 2021). Adedire et al. (2017) reported an increase in protein content from 4.41% to 5.18% with enhanced fermentation of kunu by lactic acid bacteria.
The ash content of a sample is a rough measure of its inorganic mineral elements. The proximate analysis revealed an increase in the ash content of kunu from mono- and co-cultural fermentation compared with naturally fermented samples. This increase may be due to the partial consumption of minerals by fermenting microorganisms during metabolism (Kiczorowski et al., 2022), corroborating the findings of Sanni and Adesulu (2013) who also observed an increase in ash content during the fermentation of maize for masa production using starter cultures.
The crude fibre content of kunu from mono- and co-cultural fermentation increased relative to naturally fermented kunu. This increase could be attributed to the fermenting microorganisms preferentially utilising carbohydrates and fats in the medium as energy sources, thereby leaving a higher proportion of fibre (Sumaira et al., 2022).
The total lipid content of kunu from mono- and co-cultural fermentation decreased compared with naturally fermented kunu. This reduction might be due to the utilisation of lipids by microorganisms as an energy source, sparing proteins from being used for energy (Olsen et al., 2021). These results align with those of Ndulaka et al. (2014), who observed a decrease in fat content during the production and evaluation of reconstituted kunu, although they contrast with the findings of Adedire et al. (2017), who reported an increase in fat content during enhanced fermentation of kunu.
The carbohydrate content of kunu from mono- and co-cultural fermentation decreased relative to naturally fermented kunu, possibly due to the amylolytic enzymes produced by the inoculum or the utilisation of carbohydrates as an energy source by the fermenting microorganisms, which is consistent with previous studies (Xavier & Raj, 2011; Ndukwe et al., 2023; Osman, 2011; Saleh et al., 2013).
Moisture content significantly influences the microbial flora of fermented beverages. The increase in moisture content observed in kunu from mono- and co-cultural fermentation compared with naturally fermented kunu may be due to the availability of free water, autolysis of microorganisms and the inherent high water content of the medium. Adedeji and Oluwalana (2014) reported a similar increase in moisture content in the development and quality evaluation of a non-alcoholic beverage, which concurs with the findings of Agarry et al. (2010) during the production of kunu using starter cultures.
The flavonoid and phenolic contents of kunu from mono- and co-cultural fermentation increased compared with naturally fermented kunu. This increase may result from enhanced metabolic activities of the fermenting microorganisms, and enzymes such as β-glycosidase, which hydrolyse complex phenolic compounds into simpler forms, thereby increasing the total phenolic content (Mousavi et al., 2011). Other enzymes, such as proteases, may also modify the beverage composition. These findings agree with those of Igwe et al. (2016) and Coda et al. (2012), who reported increased flavonoid and phenolic contents in kunu and other fermented beverages, respectively.
5. Conclusion
Kunu offers significant nutritional benefits that can be further enhanced by mono- and co-cultural fermentation, particularly when using S. cerevisiae and L. plantarum, rather than relying on natural fermentation alone. Mono- and co-cultural fermentation increase the protein, ash, flavonoid and phenolic contents of kunu while reducing the fat content, which is beneficial for human health. Hence, it can be concluded that mono- and co-culturally fermented kunu could serve as a good source of nutrition, as starter cultures are easier to use and offer excellent possibilities for greater control over the natural fermentation process.
Acknowledgement
We would like to express our gratitude to all those who have helped us complete this project, especially Dr. Dada from the Department of Crop, Soil and Pest Management, Federal University of Technology, Akure.
Conflict of Interest
There is no conflict of interest.
References
Abidoye, O. A. (2017). Fortification of kunun-zaki drink with cocoa powder. African Journal of Food Science, 11(4), 112–123.
Adedeji, T. O., & Oluwalana, I. B. (2014). Development and quality evaluation of a non-alcoholic beverage from cocoyam (Xanthosoma sagittifolium and Colocasia esculenta). Journal of Nigerian Institute of Food Science and Technology, 32(1), 10–20.
Adedire, O. M., Farinu, A. O., Olaoye, O. S., Osesusi, A. O., & Ibrahim, K. O. (2017). The effect of enhanced fermentation on the antioxidant, proximate and shelf-life properties of kunu. American Journal of Biological and Life Science, 5(6), 69–72.
Adeniji, P., & Ayoade, F. (2014). Screening of natural spices for improving the microbiological, nutritional and organoleptic qualities of the Zobo drink. Journal of Applied Biosciences, 76. https://doi.org/10.4314/jab.v76i1.10
Agarry, O. O., Nkama, I., & Akoma, O. (2010). Production of kunun-zaki (a Nigerian fermented cereal beverage) using starter culture. International Research Journal of Microbiology, 1(2), 18–25.
Ajagekar, A., Sali, S., & Borse, O., Patil, A., Suri, S., & Patil, A. (2023). Millets based fermented products: A review. Acta Scientific Nutritional Health, 69–81. https://doi.org/10.31080/ASNH.2023.07.1251
Amit, A. S., & Yashbir, S. S. (2024). Cereal crops: A valid option for bio-fortification and development of nutrient-dense food in developing and developed countries in Asia and Africa. In D. L. Sparks (Ed.), Advances in Agronomy, 186, 205–276. https://doi.org/10.1016/bs.agron.2024.02.010
Anyiam, P. N., Nwuke, C. P., Uhuo, E. N., Ije, U. E., Salvador, E. M., Mahumbi, B. M., & Boyiako, B. H. (2023). Effect of fermentation time on nutritional, antinutritional factors and in vitro protein digestibility of Macrotermes nigeriensis-cassava mahewu. Food Measurement, 11, 2772–2759. https://doi.org/10.1016/j.meafoo
AOAC. (2012). Official Methods of Analysis (1st ed.). Association of Official Analytical Chemists, Washington DC.
Bouakkaz, S., Zerizer, H., Rachedi, K., Accettulli, A., Racioppo, A., & Bevilacqua, A. (2024). African cereal-based fermented foods: Microbiota, functional microorganisms, starter cultures and nutritional properties. Food Bioscience, 62, 105212. https://doi.org/10.1016/j.fbio.2024.105212
Coda, R., Larena, A., Trani, A., Gobbetti, M., & Cagno, R. D. (2012). Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. International Journal of Food Microbiology, 155, 120–127.
Eduardo, L. G., Santos, B., Flores, C., Nieto, M., & Cruz, F. (2018). Low accuracy of the McFarland method for estimation of bacterial populations. African Journal of Microbiology Research, 12, 736–740. https://doi.org/10.5897/AJMR2018.8893
Erinle, B., & Ajayi, O. (2022). Bacterial contamination of vegetables sold at Oba Market, Akure, in Southwest Nigeria. GSC Advanced Research and Reviews, 13, 12–17. https://doi.org/10.30574/gscarr.2022.13.1.0246
Essien, E., Monago, C., & Edor, E. (2009). Evaluation of the nutritional and microbiological quality of kunu (a cereal-based non-alcoholic beverage) in Rivers State, Nigeria. The International Journal of Nutrition and Wellness, 10(2), 293–297.
Food and Drug Administration. (2013). Revised guidelines for the assessment of microbiological quality of processed foods. Food and Drug Administration, Philippines.
Gahalawat, P., Lamba, N., & Chaudhary, P. (2024). Nutritional and health benefits of millets: A review article. Journal of Indian System of Medicine, 12, 4–11. https://doi.org/10.1016/j.idairyj.2020.104943
Igwe, C. U., Ibegbulem, C. O., Nwaogu, L. A., Ujowundu, C. O., & Ene, A. C. (2016). Chemical composition and bioavailability of zinc and iron in kunu-zaki, a Nigerian traditional beverage. International Journal of Pharmacy, Phytochemical and Ethnomedical, 3, 9–19.
Ire, F., Edio, P., & Maduka, N. (2020). Comparative assessment of the microbiological and physicochemical quality of a laboratory brewed ‘Burukutu’ and commercialised products sold in some markets in Port Harcourt, Nigeria. EJBio, 1, 1–14. https://doi.org/10.24018/ejbio.2020.1.5.85
Jabbar, A., Tahir, M., Alhidary, I. A., Abdelrahman, M. A., Albadani, H., Khan, R. U., Selvaggi, M., Laudadio, V., & Tufarelli, V. (2021). Impact of microbial protease enzyme and dietary crude protein levels on growth and nutrient digestibility in broilers over 15–28 days. Animals, 11(9), 2499. https://doi.org/10.3390/ani11092499
Kiczorowski, P., Kiczorowska, B., Samolińska, W., Szmigielski, M., & Winiarska-Mieczan, A. (2022). Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Science Report, 12(1), 13422. https://doi.org/10.1038/s41598-022-17782
Mousavi, Z. E., Mousavi, S. M., Razavi, S. H., Emam-Djomeh, Z., & Kiani, H. (2011). Fermentation of pomegranate juice by probiotic lactic acid bacteria. Western Journal of Microbiology and Biotechnology, 27, 123–128.
Muthukumar, A., & Dakshayini, G. (2021). Morphological and biochemical characterisation: A comparative analysis of non-commercial and commercial plant growth promoting microorganisms. International Journal of Current Microbiology and Applied Sciences, 10, 867–874. https://doi.org/10.20546 /ijcmas.1002.102
Ndukwe, J. K., Aduba, C. C., Ughamba, K. T., Chukwu, K. O., Eze, C. N., Nwaiwu, O., & Onyeaka, H. (2023). Diet diversification and priming with kunu: An indigenous probiotic cereal-based non-alcoholic beverage in Nigeria. Beverages, 9(1), 14. https://doi.org/10.3390/beverages9010014
Ndulaka, J. C., Obasi, N. E., & Omeire, G. C. (2014). Production and evaluation of reconstitutable kunun-zaki. Journal of Nigerian Institute of Food Science and Technology, 32(2), 66–72.
Nwachukwu, E., Achi, O. K., & Ijeoma, I. O. (2010). Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. African Journal of Food Science and Technology, 1(2), 21–26.
Olatoye, K. K., Irondi, E. A., & Awoyale, W. (2023). Nutrient composition, antioxidant properties, and sensory characteristics of instant kunu from pearl millet supplemented with African locust bean pulp. Journal of Ethnology of Food, 10, 21. https://doi.org/10.1186/s42779-023-00188-1
Olsen, L. E. T., & Rohner, N. (2021). Lipid metabolism in adaptation to extreme nutritional challenges. Developmental Cell, 56(10), 1417–1429. https://doi.org/10.1016/j.devcel.2021.02.024
Omotade, R. O., Ganz, S., Freimüller, S., Leischtfeld, S., & Schwenninger, M. (2024). MALDI-TOF MS profiling and antifungal activity of lactic acid bacteria from kunu aya, a tiger nut traditional beverage of Nigeria. Food Bioscience, 61, 212–4292. https://doi.org/10.1016/j.fbio.2024.104581
Osman, M. A. (2011). Effect of traditional fermentation process on the nutrient and antinutrient contents of pearl millet during preparation of lohoh. Journal of the Saudi Society of Agricultural Science, 10(1), 1–6.
Saleh, A. S., Zhang, Q., Chen, J., & Shen, Q. (2013). Millet grains: Nutritional quality, processing and potential health benefits. Comprehensive Reviews in Food Science and Food Safety, 12(3), 281–295.
Sanni, A. I., & Adesulu, A. T. (2013). Microbiological and physicochemical changes during fermentation of maize for masa production. African Journal of Microbiology Research, 7(34), 4355–4362.
Senanayake, D., Torley, P. J., Chandrapala, J., & Terefe, N. S. (2023). Microbial fermentation for improving the sensory, nutritional and functional attributes of legumes. Fermentation, 9(7), 635. https://doi.org/10.3390/fermentation 9070635
Sharma, R., Maibam, B. D., & Sharma, M. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation, 6(4), 106. https://doi.org/10.3390/fermentation6040106
Sionek, B., Szydłowska, A., Küçükgöz, K., & Kołożyn-Krajewska, D. (2023). Traditional and new microorganisms in lactic acid fermentation of food. Fermentation, 9, 1019. https://doi.org/10.3390/fermentation9121019
Sumaira, J., Kumar, K., Yadav, A. N., Ahmed, N., Thakur, P., Chauhan, D., & Dhaliwal, H. S. (2022). Effect of diverse fermentation treatments on nutritional composition, bioactive components, and antinutritional factors of finger millet (Eleusine coracana L.). Journal of Applied Biology and Biotechnology, 10, 46–52. https://doi.org/10.7324/JABB.2022.10s107
Wang, Y., Wu, J., Lv, M., Shao, Z., Hungwe, M., Wang, J., Bai, X., Xie, J., Wang, Y., & Geng, W. (2021). Metabolic characteristics of lactic acid bacteria and the expanding applications in the food industry. Frontiers in Bioengineering and Biotechnology, 9, 612285. https://doi.org/10.3389/fbioe.2021.612285
Xavier, I. J. S., & Raj, S. A. (2011). Enzyme changes in rough rice during parboiling. Journal of Food Microbiology, 19(5), 381–389.
Zhina, C., Liu, T., Ye, T., Yang, X., Xue, Y., Shen, Y., Zhang, Q., & Zheng, X. (2021). Effect of lactic acid bacteria and yeasts on the structure and fermentation properties of Tibetan kefir grains. International Dairy Journal, 114, 104943. https://doi.org/10.0958/6946
Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559.
About this Article
Cite this Article
APA
Agboola, S. A., Olaniyi O. O., Adeleke, B. S., Akinyele, J. B. & Amos-Agboola, O. Y. (2025). Nutritional and Antioxidant Properties of Fermented Kunu Using Lactobacillus plantarum, Lb. fermentum, Bacillus subtilis and Saccharomyces cerevisiae Isolates. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Agboola, S. A., Olaniyi O. O., Adeleke, B. S., Akinyele, J. B. and Amos-Agboola, O. Y. 2025. “Nutritional and Antioxidant Properties of Fermented Kunu Using Lactobacillus plantarum, Lb. fermentum, Bacillus subtilis and Saccharomyces cerevisiae Isolates.” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
15 November 2024
Accepted
10 January 2025
Published
4 February 2025
Corresponding Author Email: saagboola@futa.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














