Daniel A. B. 1 , Akinyele B. J. 2 and Akinyosoye F. A. 3
1Department of Microbiology, Federal University of Technology, P.M.B. 704, Akure, Nigeria
*Corresponding Author Email:danieladenike13@gmail.com
Abstract
Aerial potato (Dioscorea bulbifera) is a perennial crop with broad leaves. The plant forms bulbils along the leaf axis of its twining stems and develops tubers beneath the ground. The aerial potato was preprocessed using various methods (peeling, boiling, parboiling, and leaving unpeeled) prior to fermentation using different techniques. The biosafety of fermented aerial potato was evaluated in Wistar albino rats using standard toxicological procedures. The results showed that the samples produced changes in the body weight of albino rats fed with fermented aerial potato; in particular, the group fed with the peeled parboiled back‐slope sample exhibited the highest percentage body weight increase (11.56%), second only to the control group (14.80%). There was also a significant increase in the blood volume of the rats. The liver hepatocytes were generally closely packed and exhibited no necrosis. Minimal focal haemorrhage was observed anteriorly, with no evidence of proliferating malignant epithelial liver cells. The hepatic acini were well formed, with no vascular damage, aggregation of lymphatic cells, or disruption of hepatic drainage, and no visible separation of the sinusoids was noted. In conclusion, the results indicate that the various treatments and fermentation processes improved the nutritional quality of the potato. Furthermore, the peeled parboiled fermented sample exhibited medicinal properties that may be useful in addressing liver problems and could potentially be harnessed to improve current commercial antibiotics.
Keywords: Aerial potato, fermentation, biosafety, toxicology, albino rats.
1. Introduction
Food fermentation is the conversion of sugars and other carbohydrates into alcohol, preservative organic acids, and carbon dioxide. All three products have found human uses. Food fermentation serves five main purposes: to enrich the diet through the development of a diversity of flavours, aromas, and textures in food substrates; to preserve substantial amounts of food through lactic acid, alcohol, acetic acid, and alkaline fermentations; to enrich food substrates with protein, essential amino acids, and vitamins; to eliminate antinutrients; and to reduce cooking time and the associated use of fuel. A number of health benefits are associated with fermentation. In fact, fermented foods are often more nutritious than their unfermented counterparts. The key health benefits of fermented foods include:
- Improves digestive health: The probiotics produced during fermentation can help restore the balance of beneficial bacteria in the gut and may alleviate some digestive problems.
- Boosts the immune system: The bacteria in the gut significantly impact the immune system. Due to their high probiotic content, fermented foods can boost the immune system and reduce the risk of infections such as the common cold. In addition, many fermented foods are rich in vitamin C, iron, and zinc, all of which contribute to a stronger immune system.
- Facilitates digestion: Fermentation helps break down nutrients in food, making them easier to digest than their unfermented counterparts. For example, lactose – the natural sugar in milk – is broken down during fermentation into simpler sugars (glucose and galactose), enabling those with lactose intolerance to consume fermented dairy products such as yoghurt and kefir.
- Reduces antinutrients: Fermentation breaks down and destroys antinutrients (e.g., phytates and lectins) found in seeds, nuts, grains, and legumes that interfere with nutrient absorption. Consequently, consuming fermented beans or legumes (such as tempeh) increases the absorption of beneficial nutrients, making them more nutritious than unfermented alternatives.
Fermented foods are generally considered safe for most people. However, some individuals may experience side effects. Due to their high probiotic content, the most common side effect is an initial, temporary increase in gas and bloating, which may be more pronounced after consuming fibre-rich fermented foods such as kimchi and sauerkraut. It is important to note that not all fermented foods are created equal; some products may contain high levels of added sugar, salt, and fat, so it is essential to read nutrition labels to ensure a healthy choice. When fermenting at home, following recipes closely is critical for safety, as incorrect temperatures, fermentation times, or unsterile equipment can lead to spoilage.
Probiotics are living bacteria and yeasts that provide health benefits when consumed in sufficient amounts. They can be taken as supplements or consumed naturally through fermented foods such as yoghurt, kefir, sauerkraut, kimchi, and kombucha. The health benefits of probiotic supplements and foods have been well documented, including a lower risk of infections, improved digestion, and even a reduced risk for some chronic diseases. Although most people do not experience side effects, the most commonly reported reaction to bacteria‐based probiotic supplements is a temporary increase in gas and bloating; those taking yeast‐based probiotics may experience constipation and increased thirst. To reduce the likelihood of side effects, it is advisable to start with a low dose of probiotics and gradually increase to the full dosage over a few weeks. When a supplement contains both probiotic microorganisms and prebiotic fibres, it is termed a synbiotic; some people may experience gas and bloating when consuming synbiotics and might prefer a supplement without prebiotics.Dioscorea bulbifera (Family: Dioscoreaceae) has been found to possess significant therapeutic potential. It is commonly used in traditional Indian, African, and Chinese medicine for the treatment of sore throat (Sharma & Bastakoti, 2009), breast cancer (Anandpara & Tirgar, 2017), and type II diabetes mellitus (Ghosh et al., 2011, 2014, 2015). Clinical trials have demonstrated that D. bulbifera is effective in treating sub‐acute thyroiditis (Jayaswori et al., 2022). Moreover, D. bulbifera has been shown to significantly induce apoptosis in HCT116 human colorectal carcinoma cells, with the ethyl acetate fraction (DBEAF) exhibiting the most compelling cytotoxicity (IC₅₀ = 37.91 ± 1.30 μg/mL; Hidayat et al., 2018) and has also demonstrated anti‐helminthic activity against Fasciola gigantica and Pheritima posthuma at a concentration of 100 mg/mL (Adeniran & Sonibare, 2013). Furthermore, D. bulbifera contains anti‐HIV-1 integrase compounds. The ethyl acetate and water fractions (1–100 mg/mL) of D. bulbifera bulbils were isolated and tested for their anti-HIV-1 integrase activity using multiplate integration. The interactions of the active compounds were investigated using molecular docking; galloyl, catechol, and sugar moieties were found to be successful inhibitors of HIV-1 integrase (Chaniad et al., 2016). Therefore, D. bulbifera has great potential in the treatment of HIV and is reported to be one of the wild foods commonly consumed by HIV patients (Nabatanzi, 2016). In addition, the methanol extract of D. bulbifera exhibits high antibacterial activity against multidrug-resistant bacteria such as Enterobacter aerogenes EA289, CM64, Klebsiella pneumoniae KP63, Pseudomonas aeruginosa PA124, Mycobacteria strain, and Staphylococcus aureus, with the lowest MIC value recorded at 16 μg/mL (Kuete et al., 2012). Its antioxidant activity in older humans has also been reported (Araghiniknam et al., 1996), with supplementation increasing serum sulphate levels and modulating lipid levels in older people. The present study aimed to evaluate the safety of fermented aerial potato.
2. Material and Methods
2.1 Collection of Aerial Potato Samples
Aerial potato tubers were obtained from a local market in Kogi State, Nigeria, where freshly harvested tubers are sold in a sterile bag and transported to the Laboratory, Department of Microbiology, Federal University of Technology, Akure. The samples were sorted to separate tubers that had been spoilt by rodent attack or physical injuries following careful examination of colour, shape, and the presence of marks and bruises. Sand was removed from the tubers by washing with sterile distilled water and air-drying to prevent spoilage. Dioscorea bulbifera is not as popular as other potato species in the study area; it is utilised mostly by rural dwellers during periods of food scarcity rather than being consumed out of preference.
2.2 Preparation of Aerial Potato Samples
The aerial potato was washed repeatedly with sterile distilled water, weighed, and divided into twelve (12) portions (A–L). Samples A–F were subjected to natural, back‐slope, and controlled fermentation, whereas samples G–L served as the non‐fermented control. After fermentation, the samples were dried at 50°C for 24 hours, cooled, milled, and packaged.
A – Unpeeled aerial potato, fermented
B – Peeled aerial potato, fermented
C – Unpeeled parboiled aerial potato, fermented
D – Peeled parboiled aerial potato, fermented
E – Unpeeled boiled aerial potato, fermented
F – Peeled boiled aerial potato, fermented
G – Unpeeled aerial potato, not fermented (control)
H – Peeled aerial potato, not fermented (control)
I – Unpeeled parboiled aerial potato, not fermented (control)
J – Peeled parboiled aerial potato, not fermented (control)
K – Peeled boiled aerial potato, not fermented (control)
L – Unpeeled boiled aerial potato, not fermented (control)
2.3 Fermentation of Aerial Potato
For all samples, 500 g of tubers were soaked in 1500 mL of tap water in a 2-litre plastic container at 27°C for 7 days. Fifty grams (50 g) of previously fermented samples (A–F) were added directly, without sterilisation, to initiate back‐slope fermentation. These were added to 450 g of raw and boiled aerial potato, and the mixture was submerged in 1500 mL of tap water in a 2-litre plastic container at 27°C for 7 days for both peeled and unpeeled samples. In a separate procedure, 500 g of aerial potato were sterilised and inoculated with starter cultures of dominant microorganisms, then fermented at 27°C for 7 days. During fermentation, microbial content and physicochemical parameters (pH measured with a handheld digital metre, total titratable acidity [TTA] measured using a titration method, and temperature measured with a mercury-in-glass thermometer) were monitored at 48-hour intervals. In addition, anti-nutrients, proximate and mineral compositions, reducing sugars, and amino and fatty acid content were analysed using standard analytical methods.
2.4 Biosafety Design
Twelve apparently healthy rats were grouped into four (4) groups of three rats each and were orogastrically administered different fermented substrates for 3 weeks. Their weight was recorded at 3-day intervals using an analytical weighing balance (capacity 1.00 kg; error margin 0.01 kg) according to the method of Conour et al. (2006). Haematological, histopathological, and biochemical analyses were carried out as follows:
2.5 Blood Collection
The anaesthetisation method involved placing cotton wool soaked in 2.00 mL of chloroform in the animal cage for 2 minutes (Adebolu et al., 2011). Blood was then collected via cardiovascular puncture and carefully transferred into EDTA bottles for haematological assays and liver function tests. For rats whose organs were not required for histopathological analysis, blood was withdrawn solely by cardiovascular puncture.
2.6 Organ Collection
For histopathological assays, organs (heart, liver, kidneys, small intestine, and spleen) were dissected from apparently healthy albino rats using dissecting tools sterilised in a hot air oven at 80°C for 3 hours. The organs were placed in specimen bottles containing 10% formalin with phosphate-buffered saline, according to the standard method described by Baker et al. (2006).
2.7 Biochemical Tests
Biochemical tests—including the determination of bicarbonate, creatinine, calcium, uric acid, and urea levels—were performed according to Baker et al. (2006) using spectrophotometry. A standardised volume (0.5 mL) of sample was pipetted onto the test zone of the appropriate test strip. The tests were carried out at 25°C, and the results were displayed on a connected computer monitor after a few seconds. All assays were performed in triplicate.
2.7.1 Liver Function Tests
Liver function tests (LFTs), including total bilirubin, serum total protein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), were performed according to Johnston et al. (1999) to assess liver function. The reagents used included phosphate buffer (100 mmol/L, pH 7.4), L-aspartate (100 mmol/L), α-oxoglutarate (2 mmol/L), and 2,4-dinitrophenylhydrazine (2 mmol/L) for AST, and 2 mmol/L AST with 200 mmol/L L-alanine for ALT. The absorbance of the sample was compared with that of a standard.
2.7.2 Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Determination
AST was determined by measuring glutamic-oxaloacetic transaminase activity via the concentration of oxaloacetate hydrazone formed with 2,4-dinitrophenylhydrazine. ALT was determined using α-oxoglutarate and L-alanine, which are converted into L-glutamate and pyruvate in the presence of alanine aminotransferase. The concentration of pyruvate formed with 2,4-dinitrophenylhydrazine was measured by recording the absorbance against a sample blank after 5 minutes at 540 nm. The activities of AST and ALT were obtained from a standard table provided by Cheesbrough (2014).
2.7.3 Alkaline Phosphatase (ALP) Determination
Serum alkaline phosphatase activity was determined using a colourless substrate, phenolphthalein monophosphate, which yields phosphoric acid and phenolphthalein that turns pink at an alkaline pH. This resultant mixture was measured photometrically.
2.7.4 Serum Total Protein Determination
Serum total protein was determined according to the method of Mokady et al. (2009). This method involves the formation of a stable complex between proteins and copper (II) ions, resulting in an alkaline pH that can be measured photometrically.
2.7.5 Serum Albumin/Globulin Ratio
Serum albumin was determined manually using a reaction mixture of bromocresol green (BCG) solution. The mixture was allowed to stand for 5 minutes, after which 0.25 mL of the solution was mixed with 0.25 mL of the sample in a glass test tube. The albumin/globulin ratio and total protein values were then determined by comparing the colour with a standard.
2.8 Haematological Tests
Haematological tests—including packed cell volume (PCV), haemoglobin concentration (Hb), red blood cell count (RBC), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and white blood cell differential count—were performed according to Cheesbrough (2006) using the following materials: blood samples, an Olympus microscope, slides, Giemsa stain, capillary tubes, a spectrophotometer, Leishman stain, EDTA bottles, and a haematocrit centrifuge. The procedures were as follows:
2.8.1 Erythrocyte Sedimentation Rate (ESR)
A Wintrobe tube was filled to the 0 mark and one end was blocked with plastocine. The tube was left standing upright, undisturbed, for 1 hour. The distance of red cell sedimentation was measured and expressed in mm per hour as the ESR.
2.8.2 Packed Cell Volume (PCV)
Blood collected into an anticoagulant bottle was mixed, and a capillary tube was filled to 75% (3/4) of its length, sealed at the outer end, and centrifuged in a micro-haematocrit centrifuge at 12,000 rpm for 5 minutes. The result was read as the percentage of packed red cells to the total volume of whole blood using a haematocrit reader.
2.8.3 Red Blood Cell Count (RBC)
The blood sample was diluted 1:200 and mixed thoroughly. A 0.02 mL aliquot was pipetted into 4 mL of diluting fluid in a bijou bottle, and the mixture was washed thoroughly by alternately drawing up and expelling the fluid. A fine Pasteur pipette was used to fill the counting chamber, and the RBCs were counted under a 40× objective.
2.8.4 White Blood Cell Count (WBC)
The blood was diluted at a ratio of 1:20, and 0.05 mL was pipetted into 0.95 mL of diluting fluid. A 0.2 mL portion was then introduced into the counting chamber and observed under a 10× objective to count the white cells per cubic millimetre.
2.8.5 Haemoglobin (Hb)
Using a mouthpiece sucker and a 0.02 mL pipette, blood was withdrawn and introduced into 4 mL of Drabkin’s solution in a tube. The tube was stoppered, mixed, and allowed to stand for 5 minutes for full colour development. A standard blood sample of known haemoglobin concentration was prepared. Using a green (624) filter, the colourimeter was calibrated to zero with plain Drabkin’s solution as a blank. The readings of the sample and the standard were recorded, and the sample haemoglobin concentration was calculated as follows:
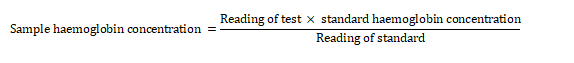
2.8.6 White Blood Cell Differential Count
Twenty microlitres (0.20 mL) of whole blood was placed on a clean, grease-free slide and spread into a thin film using a glass rod, fixed with ethanol, and stained with Giemsa stain for 5 minutes. The slide was air-dried and viewed under an oil immersion lens, according to Cougoule et al. (2012).
2.9 Histopathological Tests
Histopathological tests on vital organs (liver, heart, kidney, spleen, and intestine) were performed according to the methods of Baker et al. (2006) for sectioning, embedding, and staining, and Cheesbrough (2006) for fixation, microscopy, and interpretation.
2.9.1 Fixation
Tissue fixation was performed to prevent further enzymatic activity that leads to post-mortem autolysis. It also hardens the tissue, kills microbes, and preserves the tissue in its original form. The organs were collected and fixed in 10% buffered formalin for 24 hours; after fixation, they were trimmed to approximately 1–2 cm before dehydration.
2.9.2 Dehydration
Dehydration was performed by passing the tissue through graded concentrations of ethanol using an automatic tissue processor. The tissues were dehydrated in 50%, 70%, 80%, and 100% ethanol for 1½ hours each at 30 ± 2°C; the dehydrated tissue was then cleared using 100% xylene. The tissues were left in xylene for 2 hours to remove any residual alcohol, then placed in melted paraffin and allowed to harden for clearing. The tissues remained in molten paraffin wax for 2 hours to ensure proper embedding.
2.9.3 Sectioning
Sectioning was performed using a microtome. The tissues were sectioned to a thickness of approximately 3–10 µm and floated in a water bath at 37°C.
2.9.4 Rehydration
Rehydration was performed by passing the tissue through decreasing concentrations of ethanol to water. The tissue was passed through xylene, then through 100%, 90%, 80%, 70%, and 50% ethanol for 1½ hours at each concentration.
2.9.5 Sectioning
Staining was performed using haematoxylin and eosin (H&E), which were chosen for optimal visibility of various histological features. Haematoxylin stains the nucleus blue, while eosin stains the cytoplasm pink (acidophilic).
2.9.6 Dehydration, Mounting, and Microscopy of Stained Slides
After staining, the tissue was dehydrated again through graded concentrations of ethanol (50%, 70%, 80%, and 100%) for 1½ hours each, and cleared with xylene. Excess stain was removed using the method of Singleton (1999) under tap water. The fixed tissue on a glass slide was then mounted with Canada balsam and covered with cover slips. The preparations were left in an oven at 40°C and subsequently examined under a photomicroscope (Pagana, 2017).
3. Results
Table 1: Changes in the body weight of albino rats fed with fermented aerial potato
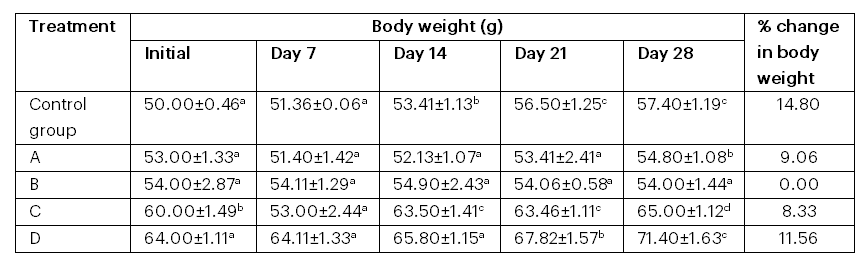
Key: A = fed with peeled parboiled fermented; B = fed with peeled back‐slope; C = fed with peeled fermented; D = fed with peeled parboiled back‐slope
Table 1 shows the changes in the body weight of albino rats fed with fermented aerial potato samples. The group fed with the peeled parboiled back‐slope sample exhibited the highest percentage increase in body weight (11.56%), after the control group (14.80%). There was no significant difference in the weight of the group fed with the peeled back‐slope fermented sample.
Table 2: Changes in the haematological parameters of albino rats fed with fermented aerial potato
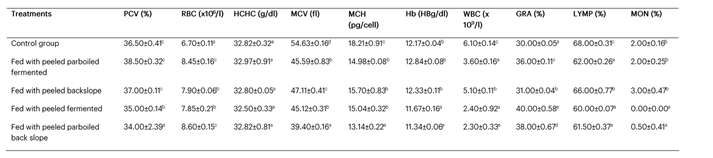
Table 2 presents the effects of the treatments on the haematological parameters of the albino rats. The greatest effect on blood volume was observed in the group fed with the peeled parboiled fermented sample, which had a PCV of 38.50 ± 0.32%.
Table 3: Changes in the organ function indices of albino rats fed with fermented aerial potato
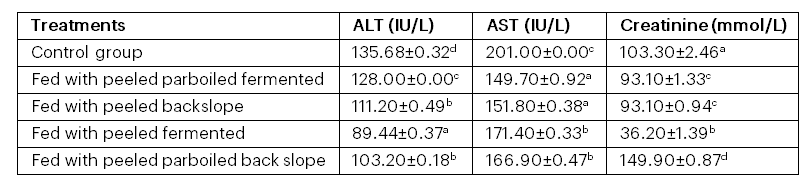
Table 3 shows the changes in the organ function indices of albino rats fed with fermented aerial potato samples. Comparatively, the treatments caused a significant (p < .05) reduction in liver function parameters (ALT and AST). A similar pattern was observed for the kidney function enzyme (creatinine), except in the rats fed with the peeled parboiled back‐slope sample, where the value was higher than that of the control group.
The histopathology results showed some pathological features in the kidney nephrons analysed. These features included the absence of necrosis, haemorrhage, or de-aggregation of lymphatic cells in the nephrons. A few visible mature convoluted tubules of nephrotic cells with numerous cell infiltrations were observed; no vascular damage was present, although a few inflammatory cell infiltrations were noted. The liver hepatocytes are generally wobbled together without necrosis. There is little atopic haemorrhage anteriorly without proliferating malignant epithelial liver cells. Their acinars are well formed, there is no vascular damage of the hepatocytes, aggregation of lymphatic cell and hepatic drainage. There is no visible separation of sinusoids.
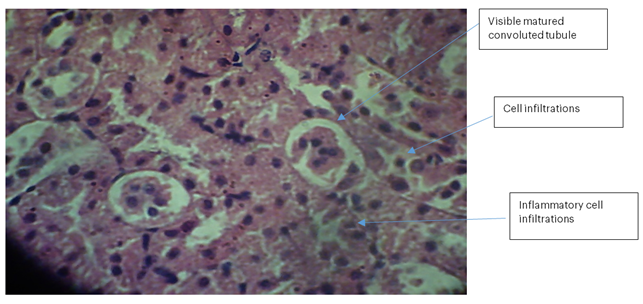
Kidney 1 (Control): No necrosis, haemorrhage, or de-aggregation of lymphatic cells was observed in the nephrons. A few visible mature convoluted tubules of nephrotic cells with numerous cell infiltrations were present, with no vascular damage but a few inflammatory cell infiltrations.
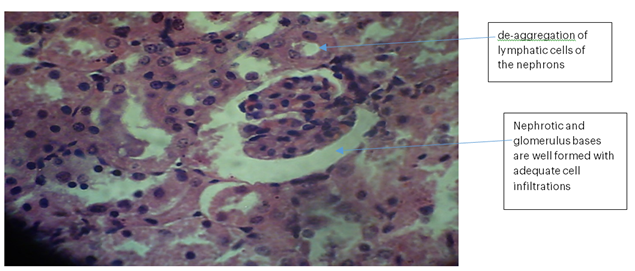
Kidney 2 (BLP): No necrosis, haemorrhage, or de-aggregation of lymphatic cells was observed in the nephrons. The nephrotic and glomerular bases were well formed with adequate cell infiltrations. There was no sign of proliferating malignant epithelial nephrotic cells; however, diminished inflammatory cells were observed.
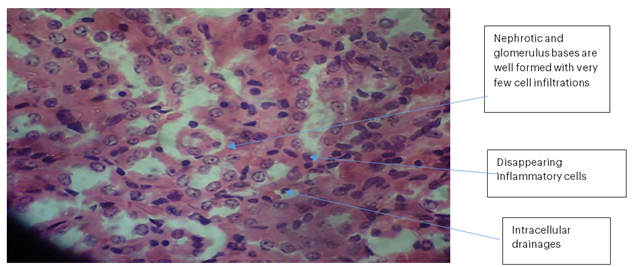
Kidney 3 (PBS): No necrosis, haemorrhage, or de-aggregation of lymphatic cells was observed in the nephrons. The nephrotic and glomerular bases were well formed with very few cell infiltrations. There were diminished inflammatory cells and expanded intracellular drainage.

Kidney 4 (PB): No necrosis, haemorrhage, or de-aggregation of lymphatic cells was observed in the nephrons. A few visible mature convoluted tubules of nephrotic cells with numerous cell infiltrations were observed; there was no vascular damage, but a few inflammatory cell infiltrations were noted.

Liver 1 (Control): The liver hepatocytes were generally closely packed without necrosis. Minimal focal haemorrhage was observed anteriorly, with no proliferating malignant epithelial liver cells. The hepatic acini were well formed, with no vascular damage, no aggregation of lymphatic cells, and intact hepatic drainage. No visible separation of the sinusoids was noted.
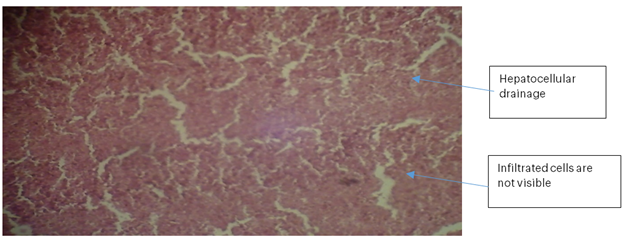
Liver 2 (BLP): The liver hepatocytes were well formed with intact hepatocellular drainage. No haemorrhage or necrosis was observed, and the sinusoids were not visibly disrupted. Infiltrated cells were not apparent, indicating active liver tissue.
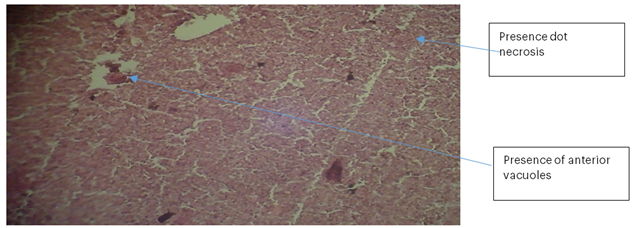
Liver 3 (PBS): The liver hepatocytes were not well formed and exhibited focal necrosis. No haemorrhage was observed, but two anterior vacuoles were present. No visible cell infiltrations or clearly defined sinusoids were noted.

Liver 4 (PB): The liver hepatocytes were well formed with active, albeit minute, sinusoids. No necrosis or haemorrhage was observed, although the hepatocellular drainage was poorly formed.
4. Discussion
The changes in body weight of albino rats fed with fermented aerial potato samples indicated that the group fed with the peeled parboiled back‐slope sample exhibited the highest percentage body weight increase (11.56%), following the control group (14.80%). This finding supports the nutraceutical properties imparted by the fermentation treatment. The lack of a significant weight difference in the group fed with the peeled back‐slope fermented sample suggests a limited improvement in nutritional value due to fermentation.
Table 2 illustrates that the treatments affected the haematological parameters of the albino rats. The highest blood volume effect was observed in the group fed with the peeled parboiled fermented sample, which had a PCV of 38.50 ± 0.32%. This may indicate the sample’s ability to stimulate haemoglobin production and its potential use in the management of anaemia.
The changes in organ function indices (Table 3) could be attributed to the functional properties of proteins, carbohydrates, and phytochemicals present in the potato (Appiah, 2010). The treatments caused a significant reduction in liver function parameters (ALT and AST), suggesting that the potato could be used for the management of liver damage. A similar trend was observed for the kidney function enzyme (creatinine), except in the rats fed with the peeled parboiled back‐slope sample, where creatinine levels were higher than in the control group; this may indicate impaired kidney integrity.
According to Adebolu et al. (2011), the increase in PCV observed in the group fed with the peeled parboiled fermented sample further supports the medicinal properties of the treated samples. The fact that some treatments produced significant weight increases and reductions in liver enzymes underscores the potential therapeutic benefits of these treatments. As noted in Stedman’s Medical Dictionary (2006), medicinal substances may function by elevating or decreasing a parameter associated with a disease condition. Therefore, the peeled parboiled fermented sample not only exhibits medicinal properties—such as improvement in PCV and reduction in liver enzymes—but may also serve as a useful substance in addressing liver and low blood conditions. Its chemical components may further be harnessed to improve current commercial chemotherapeutic agents. Among all treatments, the peeled parboiled fermented sample appears to be the most effective, as revealed by the animal bioassay.
5. Conclusion
The study confirmed the presence of Clostridium perfringens in beef samples vended at retail markets in the Akure metropolis, Ondo State. The major factors that contributed to meat contamination were low-level awareness of hygienic practices, improper handling of money, and poor sanitation of the retailer’s shops. The work revealed a 20% prevalence of C. perfringens, and the antibiotic study recorded resistance to amoxicillin and augmentin. The presence of C. perfringens alpha toxin and cpe gene was revealed in the studied beef samples. These findings indicate that beef contaminated with C. perfringens enterotoxin gene is an important risk factor for food intoxication, and food handlers that produce and process meat and meat products must have suitable food safety procedures.
The qualitative microbial risk assessment of C. perfringens confirmed in the study shows the risk of death (0.00%), the yearly risk of illness (0.999), and death per meal (0.053) obtained, which indicates that the microbial risk from C. perfringens during accidental and/or intentional consumption of beef is low. This also indicates that beef consumption may pose public health hazards; hence, hygienic procedures and practices from slaughter to consumption must be strictly adhered to.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
Adebolu, T. T., Adeoye, O. O., & Oyetayo, V. O. (2011). Effect of garlic (Allium sativum) on Salmonella typhi infection, gastrointestinal flora and haematological parameters of albino rats. African Journal of Biotechnology, 10(35), 6804–6808.
Adeniran, A. A., & Sonibare, M. A. (2013). In-vitro potential anthelmintic activity of bulbils of Dioscorea bulbifera L. on earthworms and liver flukes. Journal of Pharmacognosy and Phytotherapy, 5(12), 196–203.
Anandpara, R. C., & Tirgar, P. R. (2017). Investigation of therapeutic benefits of Dioscorea bulbifera in breast cancer. Journal of Chemical and Pharmaceutical Research, 9(6), 313–326.
Appiah, F., Oduro, I., & Ellis, W. O. (2010). Functional properties of Artocarpus altilis pulp flour as affected by fermentation. Department of Horticulture and Department of Food Science and Technology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Araghiniknam, M., Chung, S., Nelson-White, T., Eskelson, C., & Watson, R. R. (1996). Antioxidant activity of Dioscorea and dehydroepiandrosterone (DHEA) in older humans. Life Sciences, 59(11), PL147–PL157.
Baker, F. J., Breach, M. R. C. P. (2016). Modern procedures for medical laboratory science. Chris Publisher.
Cheesbrough, M. (2006). District laboratory practice in tropical countries, part 2. Cambridge university press.
Cheesbrough, M. (2014). District laboratory practice in tropical countries (2nd ed.). Cambridge University Press.
Conour, L. A., Murray, K. A., & Brown, M. J. (2006). Preparation of animals for research: Issues to consider for rodents and rabbits. ILAR Journal, 47(4), 283–293.
Cougoule, C., Van Goethem, E., Le Cabec, V., Lafouresse, F., Dupré, L., Mehraj, V., & Maridonneau-Parini, I. (2012). Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. European Journal of Cell Biology, 91(11–12), 938–949.
Ghosh, S. (2015). Phytochemistry and therapeutic potential of medicinal plant: Dioscorea bulbifera. Medicinal Chemistry, 5(4), 160–172. https://doi.org/ 10.4172/2161-0444
Ghosh, S., Ahire, M., Patil, S., Jabgunde, A., Dusane, M. B., Bimba, N., & Dhavale, D. D. (2011). Antidiabetic activity of Gnidia glauca and Dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evidence-Based Complementary and Alternative Medicine, 2012, 1–7.
Ghosh, S., More, P., Derle, A., Patil, A. B., Markad, P., Asok, A., & Dhavale, D. D. (2014). Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against α-amylase and α-glucosidase. PLOS ONE, 9(9), e107367.
Hidayat, A., Fadhlurrahman, A., Chan, C. K., Mohamad, J., & Kadir, H. A. (2018). Dioscorea bulbifera induced apoptosis through inhibition of ERK 1/2 and activation of JNK signalling pathways in HCT116 human colorectal carcinoma cells. Biomedicine & Pharmacotherapy, 104, 806–816. https://doi.org/10.101 6/j.biopha.2018.05.073
Jayaswori, S., Sabina, B., Sarbesh, R., Ramanuj, R., Young-Joo, Y., & Gaurishankar, M. (2022). Dioscorea bulbifera tuber extract causes sterility in mice. Korean Journal of Agricultural Science, 49(3), 451–462.
Johnston, D. E. (1999). Special considerations in interpreting liver function tests. American Family Physician, 59(8), 2223–2230.
Kuete, V., Teponno, B., Mbaveng, A. T., Tapondjou, L. A., Meyer, J. J. M., Barboni, L., & Lall, N. (2012). Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complementary and Alternative Medicine, 12, 1–8.
Nabatanzi, A., & Nakalembe, I. (2016). Wild food plants used by people living with HIV/AIDS in Nakisunga sub-county, Uganda. African Journal of Food, Agriculture, Nutrition and Development, 16(4), 11310–11330.
Pagana, K. T. (2017). Recent diagnostic, laboratory and histopathological test procedures (4th ed.). Morsby.
Stedman, T. L. (2006). Stedman’s medical dictionary (28th ed.). Lippincott Williams & Wilkins.
About this Article
Cite this Article
APA
Daniel A. B., Akinyele B. J. & Akinyosoye F. A.(2025). The Effects of Fermented Aerial Potato on the Blood Parameters and Selected Organs of Albino Rats. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Daniel A. B., Akinyele B. J. and Akinyosoye F. A.2025. “The Effects of Fermented Aerial Potato on the Blood Parameters and Selected Organs of Albino Rats.” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
15 November 2024
Accepted
10 January 2025
Published
4 February 2025
Corresponding Author Email: babatunde.kelly@aaua.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














