Ademosun A. O; 1 Ojueromi O. O. 2 Ayodejia N. F.; 3 & Obohs G. 4
1Functional Foods and Nutraceuticals Unit, Department of Biochemistry,
Federal University of Technology, Akure, Ondo State, Nigeria.
2Department of Pure and Applied Sciences, Precious Cornerstone University,
Ibadan, Oyo State, Nigeria.
*Corresponding Author Email: …
Abstract
Tiger nut (Cyperus esculentus) and date (Phoenix dactylifera) are bioactive‐rich foods employed in the production of functional food products based on their health benefits, particularly their antioxidant properties. This study sought to evaluate the antioxidant indices and oxidative stress markers in the rat brain following administration of tiger nut and date‐enriched pancakes. The experimental animals were distributed into seven groups of five rats each. The rats were fed pancakes formulated with tiger nut and date at varying proportions [(25 g tiger nut + 15 g date; 50 g tiger nut + 15 g date; 75 g tiger nut + 15 g date; 100 g tiger nut + 15 g date] for 14 days. The antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)] and oxidative stress markers [malondialdehyde (MDA) and reactive oxygen species (ROS)] were evaluated. The results showed that the ROS and MDA levels in the brains of rats fed the tiger nut and date‐formulated pancake were significantly reduced (p < .05), with a 25–41% reduction in MDA levels compared with the control group. Consequently, a significant (p < .05) increase in the activities of SOD, CAT, GPx and glutathione‐S‐transferase (GST) was observed in the groups fed the tiger nut and date‐enriched pancake. Supplementation with a pancake containing 100 g tiger nut and 15 g date exhibited the best antioxidant and oxidative stress–lowering effect. This study reveals that dietary inclusion of tiger nut and date may protect against oxidative stress and enhance the in vivo antioxidant status in rats. Therefore, tiger nut and date could be used in the production of antioxidant‐rich functional food products.
Keywords: Antioxidant properties, oxidative stress, tiger nut, date fruit, functional food.
1. Introduction
Free radicals are reactive molecules or atoms with unpaired electrons that can cause rapid reactions, destabilising other molecules and creating more free radicals (Di Meo & Venditti, 2020). The body combats these free radicals through antioxidative mechanisms. In the brain, where oxygen consumption is high, neurons produce reactive oxygen species (ROS), including superoxide anions, through mitochondrial activity. Antioxidants help neutralise both free radicals and ROS, reducing their harmful impact on cells. These antioxidants function as inhibitors of oxidation even at relatively low concentrations and consequently play various physiological roles. The antioxidative system comprises both enzymatic and non-enzymatic components. The non-enzymatic system includes ascorbic acid (vitamin C), α-tocopherol, carotenes, etc., while the enzymatic system includes superoxide dismutase, catalase and peroxidase (Srivastava, 2020). The function of this antioxidant system is to scavenge the toxic radicals produced during oxidative stress.
The utilisation of fruits, tubers, spices and herbs in culinary practices as therapeutic agents is a prevailing trend that has gained global acceptance. The intake of antioxidant compounds present in food is an important health-protecting factor. Natural antioxidants from foods and other biological materials have attracted considerable interest because of their presumed safety and potential nutritional and therapeutic effects. Numerous plant sources have been evaluated for antioxidants due to the growing interest in finding natural alternatives to synthetic antioxidants. Tiger nut (Cyperus esculentus L.) and date (Phoenix dactylifera L.) are consumed widely either individually, mixed together or paired with other fruits, seeds and vegetables. Tiger nuts have a rich phytochemical profile composed of flavonoids, organic acids, alkaloids, glycosides (Metsämuuronen & Sirén, 2019), monounsaturated fatty acids, tannins, phytates and oils. Polyphenolic compounds, such as flavonoids, possess strong antioxidant properties.
This study aims to provide scientific evidence regarding the antioxidative potential of tiger nut and date–formulated pancakes. The findings may contribute to the development of novel functional foods that harness the inherent antioxidant properties of tiger nut and date, offering a practical and palatable solution for reducing oxidative stress and promoting health.
2. Material and Methods
2.1 Sample Collection
Yellow Tiger nut (Cyperus esculentus), white maize (Zea mays), white millet (Panicum miliaceum), white sorghum (Sorghum bicolour), Coconut (Cocos nucifera) and Ginger (Zingiber officinale) samples were purchased from King’s market in Ado-Ekiti, Ekiti state, Nigeria and transported to the laboratory for further analyses. All samples were sorted, cleaned and kept in sterile containers until needed.
2.1.1 Chemicals and Reagents
Chemicals and reagents such as thiobarbituric acid (TBA), tetraoxosulphate(VI) acid (H₂SO₄), DNPH, saline, trichloroacetic acid (TCA), hydrogen peroxide, vitamin C standard, phosphate buffer, sodium azide, Ellman’s reagent, methanol, acetic acid, FeSO₄, potassium dichromate and glutathione were obtained from analytical suppliers. All chemicals and reagents were of analytical grade, and the water used was glass-distilled.
2.2 Preparation of Pancake
Tiger nuts and dates were washed thoroughly, peeled, cut into small pieces and oven-dried at 40°C for 48 hours. The dried pieces were milled into a fine powder and kept in an air-tight container. The pancake was prepared by mixing the appropriate proportions (as shown below) of dried tiger nut and date powders with flour, egg albumin and water. The mixture was thoroughly blended until smooth and well combined, then baked in a frypan on a gas cooker at medium heat.
2.3 Experimental Design
Male albino rats (180–200 g) were purchased from the Animal House, Department of Biochemistry, Federal University of Technology, Akure, Nigeria. The rats were maintained at 25°C with free access to food and water and were acclimatised under these conditions for two weeks prior to the commencement of the experiments. The rats were fed pancakes supplemented with tiger nut and date at varying dietary inclusions for 14 days. The experimental procedures were endorsed by the Federal University of Technology Akure Committee for the Ethical Use of Research Animals (CERAD, FUTA).
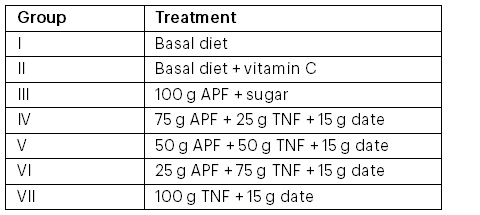
Note: APF = all-purpose flour; TNF = tiger nut flour.
The experiment lasted for 14 days, after which the animals were decapitated following 24 hours of fasting by cervical dislocation. The brain was isolated, rinsed in cold saline (0.9%) and homogenised in phosphate buffer.
2.3.1 Preparation of Tissue Homogenate
The rats were decapitated under mild diethyl ether anaesthesia, and the brain was rapidly isolated, placed on ice and weighed. The tissue was rinsed in cold saline (1:10 w/v) and homogenised in sodium phosphate buffer (0.1 M, pH 6.9) using approximately 10 up-and-down strokes at approximately 1200 rev/min in a Teflon-glass homogeniser. The homogenate was centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant was used for subsequent biochemical assays.
2.4 Biochemical Assays
2.4.1 Estimation of Lipid Peroxidation
The lipid peroxidation assay was carried out using a modified method of Ohkawa et al. (1979). Briefly, 300 µL of tissue homogenate, 300 µL of 8.1% SDS (sodium dodecyl sulphate), 500 µL of acetic acid/HCl (pH 3.4) and TBA were added. The mixture was incubated at 100°C for 1 hr. Thereafter, the thiobarbituric acid reactive species (TBARS) produced were measured at 532 nm and calculated as malondialdehyde (MDA) equivalents.
2.4.2 Determination of Reactive Oxygen Species (ROS) Concentration
ROS levels were determined using the method of Hayashi et al. (2007). Briefly, 50 µL of tissue homogenate and 1400 µL of sodium acetate buffer (0.1 M, pH 4.8) were transferred into a cuvette and the reagent mixture added. The absorbance was measured at 505 nm using a spectrophotometer.
2.4.3 Catalase (CAT) Activity
CAT activity was determined in accordance with a modified method of Nelson and Kiesow (1972). This assay involves measuring the change in absorbance at 240 nm due to the CAT-dependent decomposition of hydrogen peroxide. Enzymatic activity was expressed in units per mg protein (one unit of the enzyme decomposes 1 mmol of H₂O₂ per min at pH 7 and 25°C).
2.4.4 Superoxide Dismutase (SOD) Activity
SOD activity was determined in tissue homogenates by measuring the inhibition of the autoxidation of epinephrine at pH 10.2 and 30°C according to Misra and Fridovich (1972). Enzymatic activity was expressed in units per mg protein.
2.4.5 Estimation of Glutathione Peroxidase (GPx) Activity
GPx activity was determined according to the method of Rotruck et al. (1973).
2.4.6 Estimation of Glutathione Transferase (GST) Activity
GST activity was determined following the method of Mannervik and Guthenberg (Mannervik et al., 1981). The absorbance of the reaction mixture was measured at 340 nm after a 5‐min incubation and expressed as U/g of protein.
2.4.7 Total Protein Determination
Protein content of the tissue homogenate was determined using the Bradford (1976) method. Briefly, 50 µL of homogenate was mixed with 2500 µL of Coomassie Blue reagent, incubated at room temperature for 30 min, and the absorbance was read at 595 nm.
3. Result
This study examined the effects of tiger nut and date–formulated pancakes on oxidative stress markers, including ROS and MDA, in experimental rats. All groups treated with dietary supplementation of tiger nut and date [25 g tiger nut + 15 g date; 50 g tiger nut + 15 g date; 75 g tiger nut + 15 g date; 100 g tiger nut + 15 g date] showed a marked decrease in brain ROS and MDA levels compared with the control group and the group fed the sugar-formulated pancake (see Figures 1 and 2).
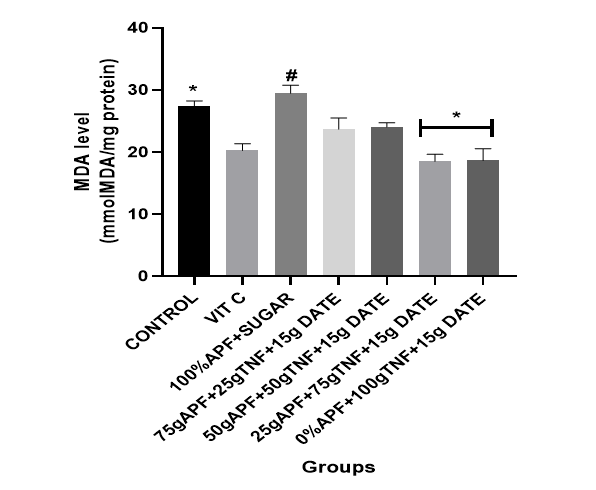
Figure 1: The effect of tiger nut and date- enriched pancake on the brain’s malondialdehyde (MDA) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date; 25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
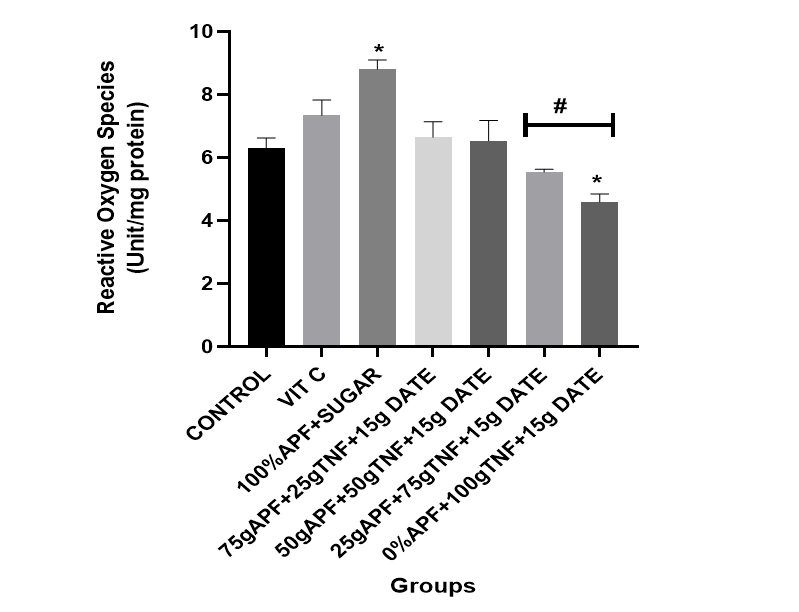
Figure 2: The effect of tiger nut and date- enriched pancake on the brain’s reactive oxygen species (ROS) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date; 25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
Conversely, the antioxidant enzyme activities (SOD, CAT, GPx and GST) were significantly (p < .05) lower in the control group (by 25–41%) (see Figures 3–6).
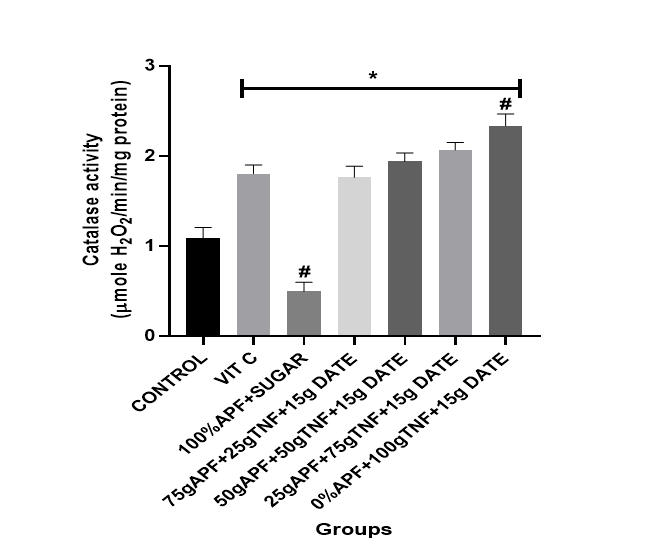
Figure 3: The effect of tiger nut and date- enriched pancake on the brain’s catalase (CAT) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date; 25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
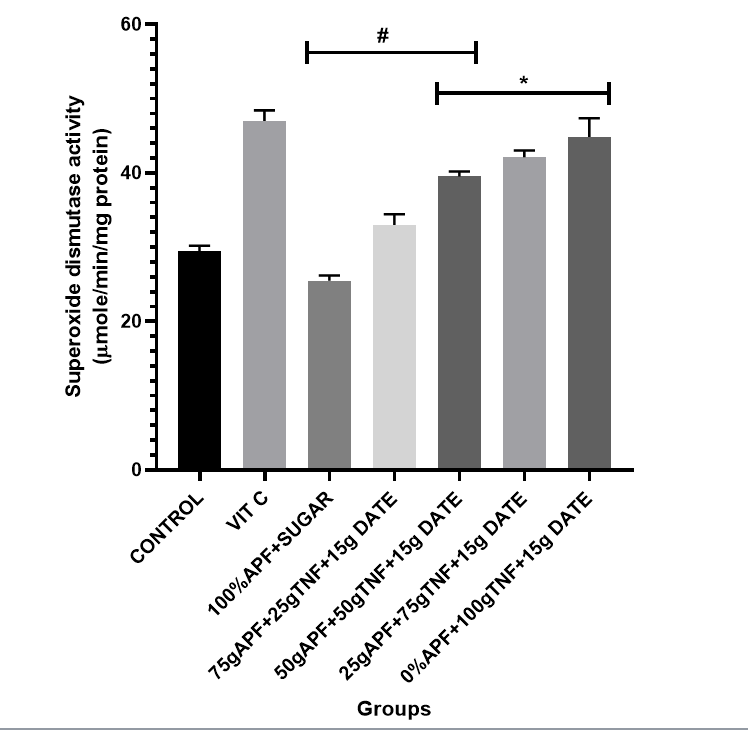
Figure 4: The effect of tiger nut and date- enriched pancake on the brain’s superoxide dismutase (SOD) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date;25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
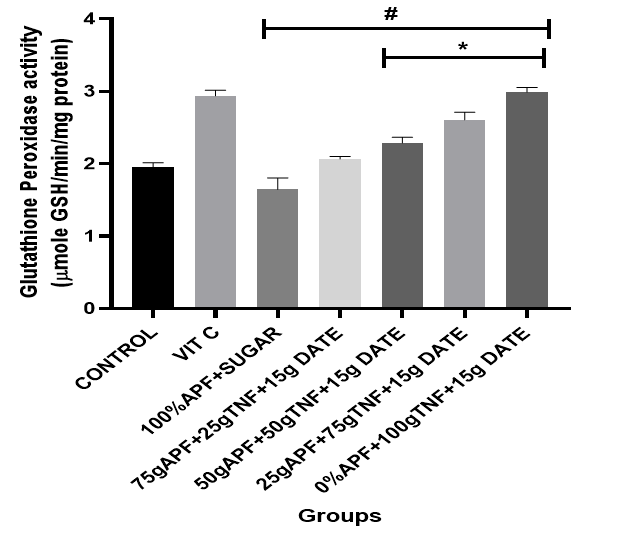
Figure 5: The effect of tiger nut and date- enriched pancake on the brain’s glutathione Peroxidase (GPX) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date; 25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
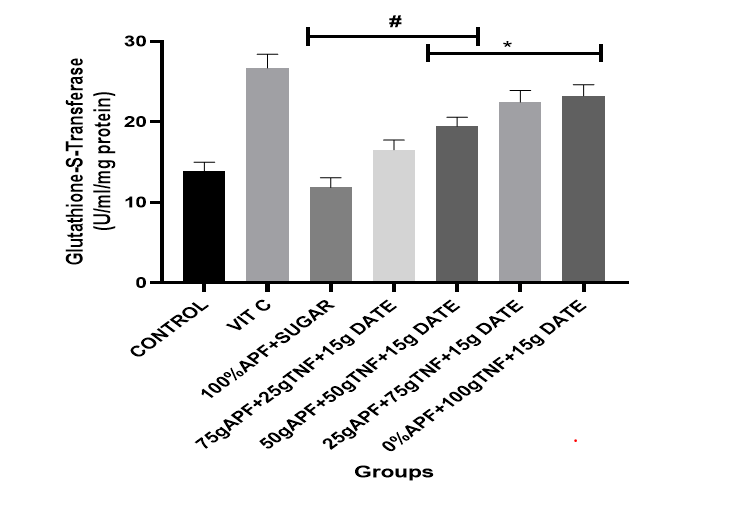
Figure 6: The effect of tiger nut and date- enriched pancake on the brain’s glutathione-Transferase (GST) level in rats.
Bars are expressed as mean ± standard error of the mean (SEM) (n=5). *P < 0.05 versus Control group and #P < 0.05 versus Vitamin C
Keys: Vit C= Vitamin C; 100% APF+ Sugar=100% All Purpose Flour+ Sugar; 75gAPF+25gTNF +Sugar=75g All Purpose Flour +25g Tiger nut Flour+15g of Date; 50gAPF+50gTNF +Sugar=50g All Purpose Flour +50g Tiger nut Flour+15g of Date; 25gAPF+75gTNF +Sugar=25g All Purpose Flour +75g Tiger nut Flour+15g of Date; 0gAPF+100gTNF +Sugar=0g All Purpose Flour +100g Tiger nut Flour+15g of Date
However, treatment with the tiger nut and date–formulated diet at varying dietary inclusions resulted in a considerable elevation in SOD, CAT, GPx and GST levels. Notably, treatment with pancakes formulated with 75 g tiger nut + 15 g date and 100 g tiger nut + 15 g date produced a marked elevation in antioxidant indices [CAT (74–93%); SOD (34–41%)].
4. Discussion
Dietary control is a practical approach for the prevention and management of oxidative stress, neurological disorders and chronic degenerative diseases (Ademosun et al., 2023). Plant-based diets boost antioxidant activity in living organisms (Ademiluyi, et al., 2019). Date and tiger nut are regarded as nutrient-rich, antioxidant-packed, plant-based foods due to their high polyphenolic content, such as flavonoids, which have traditionally been utilised for their therapeutic properties. They contain a variety of bioactive phytochemicals. Oxidative stress and free radicals are generally known to be detrimental to human health (Pizzino, et al., 2017). Numerous studies have demonstrated that free radicals contribute to the initiation and progression of several pathologies, ranging from cardiovascular diseases to cancer (Ademosun et al., 2023). Antioxidants—compounds capable of counteracting oxidative stress—have gained enormous attention from the biomedical research community, as they have demonstrated significant efficacy in disease prevention and/or treatment with minimal or no deleterious side effects. Oxidative stress occurs when free radicals outweigh the antioxidant defence system (Oboh, et al., 2014; Ojueromi, et al., 2022). It is a key contributing factor to neurodegeneration in various adult-onset neurological conditions, including Alzheimer’s disease and amyotrophic lateral sclerosis, as it causes damage to cellular components. ROS, the primary indicator of oxidative stress examined in this study, are radicals with unpaired electrons in their outermost orbital; they play roles in cell signalling but are also by-products of deleterious oxidation reactions (Fadaka, et al., 2019). Although ROS are produced by normal cellular metabolism and are important in signalling, gene expression, cell proliferation and homeostasis (Ogunsuyi, et al., 2020), excessive ROS production can be damaging. The breakdown of polyunsaturated fatty acids by free radicals leads to the formation of TBARS, with MDA being one of the most popular biomarkers for lipid peroxidation (Oboh, et al., 2017).
Elevated MDA indicates increased ROS levels, which can damage biological tissues, disrupt biochemical compounds and compromise cell membrane integrity. The results of this study showed a significant reduction in ROS and MDA levels in the brains of rats fed the tiger nut and date–formulated pancake compared with the control and the group fed a sugar and all-purpose flour (APF) pancake. This suggests that the tiger nut and date–formulated pancake possess antioxidant-boosting capacity, consistent with the findings of Adedayo, et al. (2015) and Alabi, et al. (2024). These findings may have therapeutic significance in controlling oxidative stress by reducing free radical-induced damage.
Another important mechanism by which organisms mitigate ROS damage is via enzymatic antioxidants such as SOD, CAT and GPx. SOD catalyses the conversion of superoxide radicals to hydrogen peroxide, while CAT converts hydrogen peroxide into water and oxygen (Ademosun et al., 2023; Ojueromi, et al., 2024). Glutathione‐S‐transferase (GST) facilitates the binding of reduced glutathione (GSH) to electrophilic compounds, forming a thioester bond between the sulphur atom of GSH and the substrate. Intracellular GST levels are an important biomarker for tissue injury (Abolaji, et al., 2016). GST detoxifies xenobiotics and neutralises intracellular peroxides and electrophilic oxidants, thereby providing protection against oxidative stress. Previous studies have reported that the conversion of the superoxide anion by SOD, followed by the conversion of less-damaging compounds to water by CAT, is crucial for lifespan extension (Oyeniran, et al., 2021; Ojueromi, et al., 2024). The present study found a significant elevation in the activities of SOD, CAT, GPx and GST in rats treated with the formulated pancake.
Furthermore, GPx is an antioxidant enzyme that reduces various organic and inorganic hydroperoxides to their corresponding hydroxyl forms using glutathione, thereby playing a crucial role in protecting cells from oxidative damage (Kühn & Borchert, 2002). Thus, the increased GPx activity observed in groups fed the formulated pancakes indicates an enhanced capacity to scavenge free radicals, which in turn helps to prevent lipid peroxidation and maintain intracellular homeostasis and redox balance.
5. Conclusion
This study demonstrates that tiger nut and date–formulated pancakes can significantly enhance the brain’s antioxidant defence system, offering potential applications as functional foods for mitigating oxidative stress and reducing the risk of neurodegenerative diseases. However, further research, including clinical trials, is recommended.
Conflict of Interest
The authors declare that they have no conflicts of interest related to this manuscript.
References
Ademosun, A., Ojueromi, O., Peace, O., & Oboh, G. (2023). Cardiomodulatory and antioxidative potentials of almond–citrus peel fortified shortbread in high fat diet/L-NAME-induced hyperlipidaemic–hypertensive rats. Journal of Medicinal Food, 26(8), 586–594.
Abolaji, A. O., Adedara, I. A., Abajingin, A. O., Fatunmibi, O. J., Ladipo, E. O., & Farombi, E. O. (2016). Evidence of oxidative damage and reproductive dysfunction accompanying 4-vinylcyclohexene diepoxide exposure in female Wistar rats. Reproductive Toxicology, 66, 10–19.
Adedayo, B. C., Agunloye, O. M., Obawarrah, R. Y., & Oboh, G. (2024). Caffeic acid attenuates memory dysfunction and restores the altered activity of cholinergic, monoaminergic and purinergic systems in the brain of cadmium chloride–exposed rats. Journal of Complementary and Integrative Medicine, 21(2), 230–238.
Alabi, A. O., Adeoti, O. A., Azeez, L. A., & Olatunji, B. F. (2024). Effect of refrigerated storage on the quality, antioxidant and sensory properties of functional “OGI” enriched with dietary tigernut (Cyperus esculentus L.) fibre emulsion. European Journal of Nutrition & Food Safety, 16(1), 95–111.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72, 248–254.
Di Meo, S., & Venditti, P. (2020). Evolution of the knowledge of free radicals and other oxidants. Oxidative Medicine and Cellular Longevity, 2020, Article 9829176
Fadaka, A. O., Ajiboye, B. O., Adewale, I., Ojo, O. A., Oyinloye, B. E., & Okesola, M. A. (2019). Significance of antioxidants in the treatment and prevention of neurodegenerative diseases. Journal of Phytopharmacology, 8(2), 75–83.
Mannervik, B., & Guthenberg, C. (1981). Glutathione transferase (human placenta). In Methods in Enzymology (Vol. 77, pp. 231–235).
Misra, H. P., & Fridovich, I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biology and Chemistry, 247(10), 3170–3175.
Kühn, H., & Borchert, A. (2002). Regulation of enzymatic lipid peroxidation: The interplay of peroxidising and peroxide-reducing enzymes. Free Radical Biology and Medicine, 33(2), 154–172.
Oboh, G., Ogunruku, O. O., Oyeleye, S. I., Olasehinde, T. A., Ademosun, A. O., & Boligon, A. A. (2017). Phenolic extracts from Clerodendrum volubile leaves inhibit cholinergic and monoaminergic enzymes relevant to the management of some neurodegenerative diseases. Journal of Dietary Supplements, 14(3), 358–371.
Ogunsuyi, O. B., Oboh, G., Oluokun, O. O., Ademiluyi, A. O., & Ogunruku, O. O. (2020). Gallic acid protects against neurochemical alterations in a transgenic Drosophila model of Alzheimer’s disease. Advances in Traditional Medicine, 20, 89–98.
Ojueromi, O. O., Oboh, G., & Ademosun, A. O. (2022). Black seed (Nigella sativa): A favourable alternative therapy for inflammatory and immune system disorders. Inflammopharmacology, 30(5), 1623–1643.
Ojueromi, O. O., Oboh, G., & Ademosun, A. O. (2024). Nigella sativa-fortified cookies ameliorate oxidative stress, inflammatory and immune dysfunction in a Plasmodium berghei-infected murine model. Journal of Medicinal Food, 27(6), 552–562.
Oyeniran, O. H., Ademiluyi, A. O., & Oboh, G. (2021). African mistletoe (Tapinanthus bangwensis Lor.) infestation improves the phenolic constituents, antioxidative and antidiabetic effects of almond (Terminalia catappa Linn.) host leaf in sucrose‐rich diet‐induced diabetic-like phenotypes in fruit fly (Drosophila melanogaster Meigen). Food Frontiers, 2(1), 77–90.
About this Article
Cite this Article
APA
Ademosun A. O., Ojueromi O. O., Ayodeji N. F., & Obohs G. (2025). Tiger Nut (Cyperus esculentus) and Date (Phoenix dactylifera) Enriched Pancakes Improve Antioxidant Status in Rat Brain. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Ademosun A. O., Ojueromi O. O., Ayodeji N. F., and Obohs G. 2025. “Tiger Nut (Cyperus esculentus) and Date (Phoenix dactylifera) Enriched Pancakes Improve Antioxidant Status in Rat Brain” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
03 November 2024
Accepted
7 January 2025
Published
4 February 2025
Corresponding Author Email: olawale.odeleye@fuoye.edu.ng
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.














