EGabriel-Ajobiewe R.A.O;1 Ademoyegun S.O;2 Adeleke B.S.;3 & Bankefa E.O. 4
1Department of Microbiology, Federal University Oye-Ekiti, Ekiti State, Nigeria.
2Department of Biological Sciences, Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State, Nigeria.
*Corresponding Author Email: adefolakemi.gabriel-ajobiewe@fuoye.edu.ng; microbade@yahoo.com …
Abstract
The growing demand for non‐dairy probiotic products has prompted renewed interest in utilising fruit‐based beverages and juices as vehicles for health‐promoting microorganisms. In this study, lactic acid bacteria (LAB) were isolated from kefir and Ogi, and subsequently characterised both phenotypically and molecularly. Orange and pineapple juices were extracted using a hand‐operated steel juice extractor, pasteurised and stored at 4 °C. The identified LAB were inoculated into the pasteurised juices in two distinct categories using standard methods. The shelf‐life of the juices was determined with the aid of natural and artificial preservatives, and the physicochemical parameters of the pasteurised, probiotified and preserved juices were analysed. Total bacterial counts before and after probiotification indicated that the cocktail juice recorded the highest counts, with 4.96 × 10⁵ CFU/mL in unpasteurised samples and 1.77 × 10⁴ CFU/mL in pasteurised samples. Lactobacillus spp. were identified as the predominant bacteria in the juice samples. High pH values of 3.95 and 3.78 were recorded for the pasteurised orange and cocktail juices, respectively, while no significant effect on sample temperature was observed following probiotification. Moisture content was highest in the pasteurised cocktail juice (93.65 g/100 g), whereas the probiotic orange juice exhibited the greatest ash content (0.53 g/100 g). Protein composition was also enhanced by probiotification, with the probiotic orange juice containing 3.57 g/100 g compared to 0.58 g/100 g in the pasteurised orange juice. These findings suggest that fortifying fermenting fruit juices with selected Lactobacillus spp. can significantly enhance food quality and safety.
Keywords: Fermenting juice, food fortification, Lactobacillus spp., non‐alcoholic beverages, organic acid.
1. Introduction
The consumption of live microorganisms is increasingly recommended by medical specialists for the prevention and treatment of various illnesses, as well as for the regulation of digestive and immunological health (Hill et al. 2014; Sanders et al. 2018). Among the various probiotic microorganisms, species of Lactobacillus and Bifidobacterium have been widely incorporated into foods, not only for their preservative and fermentative capabilities but also for the resultant health benefits (Hossain et al. 2019). The shift towards non‐dairy probiotic products is largely driven by factors such as vegetarianism, lactose intolerance, concerns over milk cholesterol content and milk protein allergies. This trend underscores the potential of fruit‐based beverages and juices as effective probiotic carriers.
LAB are particularly valued for their ability to produce organic acids and bacteriocins, which exhibit antibacterial, antiviral and antioxidant properties (Fazeli et al. 2011). The isolation and use of selected LAB as starter cultures in food production processes are critical to ensuring food safety and supporting sustainable human health. Oranges (Citrus sinensis), renowned for their rich nutritional and vitamin content, are predominantly grown in the tropics and serve as a major dietary resource (Etebu et al. 2008). Similarly, pineapple (Ananas comosus) is one of the most extensively produced tropical fruits, with countries such as Nigeria, Mexico and Indonesia ranking among its principal producers (Carlier et al. 2014). A juice cocktail, typically comprising a blend of fruit juices (e.g., orange, pineapple) and occasionally other ingredients such as water or sugar, represents a versatile non‐alcoholic beverage option.
The beneficial effects of fermented dairy products containing LAB on intestinal microflora—often termed probiosis—have been well documented. The presence of viable LAB in the gastrointestinal tract can directly influence the composition of the microbial community (Gasbarrini et al. 2016; Anadon et al. 2021). Historically, the health benefits of probiotics were first systematically investigated by Metchnikoff, whose work laid the foundation for subsequent research in this field.
In Africa, traditional fermented foods such as Ogi—a cereal‐based product commonly prepared from sorghum, maize, or millet—serve as important dietary staples (Olaniran et al. 2020; Olaniran & Abiose 2019; Greppi et al. 2013; Omemu 2011; Adelekan & Oyewole 2010). LAB, particularly species of Lactobacillus, have been implicated in the fermentation of cereals to produce Ogi (Zheng et al. 2020; Olaniran et al. 2020). Similarly, kefir—a fermented milk product known for its diverse microbial content—is reputed for its health benefits, including improvements in digestive health and immune function (Otles & Cagindi 2003; Chaitow & Trenev 2002).
The shelf‐life of probiotic‐enhanced juices can be further extended through the use of natural substances, such as essential oils, which are generally recognised as safe (GRAS) and exhibit antibacterial properties against bacteria, yeasts and moulds (Speranza & Corbo 2010). Certain plant essential oils have also been documented to exert antifungal, anti‐aflatoxigenic and antioxidant activities (Prakash et al. 2012; Soliman & Badeaa 2002; Farag et al. 2006; Jaya & Dubey 2011; Tomaino et al. 2005). Although the probiotification of fermented fruit juices using LAB has been explored, there remains a paucity of comprehensive studies in this area. Therefore, this study was designed to evaluate the effect of LAB as a tool for the probiotification of orange and cocktail juices, with an emphasis on changes in composition and quality.
2. Materials and Methods
2.1 Sample Collection
Five grams of kefir starter culture were procured from Pentmark Global Resources, Lagos, Nigeria. Locally produced maize Ogi was obtained from Akure, Ondo State, Nigeria. Lemons and limes were purchased from Oye Market and transported to the microbiology laboratory for subsequent analysis.
2.2 Microbial Analysis
Viable cell counts were determined by serial dilution (up to 10⁷) of the samples. Aliquots of 0.1 mL from the 10³ and 10⁵ dilutions were plated in triplicate onto Petri dishes containing nutrient agar, followed by incubation at 37 °C for 24 h. Colonies were enumerated and recorded as colony‐forming units (log CFU/mL).
2.3 Isolation and Characterisation of Lab
LAB were isolated from both the kefir starter and the locally produced Ogi using standard microbiological techniques. After 24 h of incubation at 37 °C, pure cultures were obtained and subjected to morphological and biochemical characterisation. Biochemical tests included Gram staining, citrate utilisation, catalase activity, indole production, motility, spore formation, oxidase activity and glucose fermentation.
2.4 Molecular Characterisation of Lab Isolates
Molecular identification of the LAB isolates was conducted using modified methods as described by Hidayat (2019). DNA was extracted from pure cultures and subjected to polymerase chain reaction (PCR) amplification, followed by sequencing and comparison with existing databases for accurate identification.
2.5 Production of the Juices
Fresh oranges and pineapples were purchased from a local fruit market in Ado‐Ekiti, Nigeria. The fruits were thoroughly washed to remove surface dirt and allowed to air dry. For the production of orange juice, 20 oranges per treatment were manually squeezed using a sterilised hand‐operated steel juice extractor (Zumex Z38, Valencia, Spain). The extracted juice was immediately stored at 4 °C. Similarly, medium‐sized pineapples (five per treatment) were processed and stored at 4 °C. Orange juice was maintained separately from the cocktail juice, which comprised a mixture of orange and pineapple juices (Bagci & Temiz 2011).
2.5.1 Probiotification of the Juices
Pasteurised juices were inoculated with the characterised LAB using a modified method described by Cheesbrough (2002). Two categories were prepared: probiotic orange juice and probiotic cocktail juice.
2.5.2 Extraction of Lemon and Lime Essential Oils
Essential oils were extracted from lemon and lime peels by adapting the methods of Al-Jabri and Hossain (2018). The peels were subjected to simple distillation using a Soxhlet apparatus. The resultant distillate, comprising two distinct layers (oil and water), was separated using a separating funnel. The extracted oils were stored in sample bottles at freezer temperatures until required.
2.6 Shelf-Life Determination using Natural and Artificial Preservatives
Shelf-life studies were performed using modified methods of Ramachandran et al. (2017). Probiotic juices were aliquoted into eight groups (25 mL per sample) and labeled as follows:
- Probiotic Orange Juice Blank (POJ)
- Probiotic Orange Juice with Sodium Sulphite (POJSs): 1.5 mg of sodium sulphite (Na₂SO₄) in 25 mL of juice (corresponding to 60–80 mg/L)
- Probiotic Orange Juice with Lemon Essential Oil (POJLO): 1 mL of 1% lemon essential oil per 25 mL of juice
- Probiotic Orange Juice with Lime Essential Oil (POJLiO): 1 mL of 1% lime essential oil per 25 mL of juice
- Probiotic Cocktail Juice Blank (PCJ)
- Probiotic Cocktail Juice with Sodium Sulphite (PCJSs)
- Probiotic Cocktail Juice with Lemon Essential Oil (PCJLO)
- Probiotic Cocktail Juice with Lime Essential Oil (PCJLiO)
2.7 Physicochemical Analysis
A portable digital pH meter (FiveGo™ F2 Standard) was employed to measure the pH of the juice samples. Total soluble solids (°Brix) were determined using a portable refractometer (Salinity). Temperature measurements were recorded using a BSI‐BS 593 thermometer. Total acidity, expressed as a percentage of lactic acid, was determined by titrating 10 mL of each juice sample with 0.1 N NaOH to an endpoint of pH 8.2 ± 0.1. Additional compositional analyses, including moisture content, ash content and protein levels, were performed using standard methods.
3. Results
3.1 Microbial Counts
Table 1 presents the total viable count (TVC) of bacterial isolates obtained from the Ogi and kefir starter cultures. The kefir starter culture recorded a significantly higher bacterial count of 4.05 × 10⁴ CFU/mL compared to the Ogi culture, which exhibited a lower count of 3.0 × 10⁴ CFU/mL. In Table 2, the total viable plate counts of bacteria and lactic acid bacteria (LAB) in each juice sample, both before and after probiotification, are displayed. Notably, the probiotic cocktail juice exhibited the highest LAB count at 5.50 × 10⁴ CFU/mL, followed by the unpasteurised cocktail juice at 4.96 × 10⁵ CFU/mL, and the pasteurised orange juice recorded the lowest count at 1.46 × 10⁴ CFU/mL.
Table 1: Total Viable Count of Bacterial Isolates from Ogi and Kefir Starter Culture
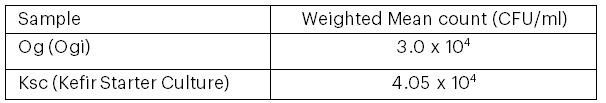
Note: This table represents the mean total viable count of bacterial isolates.
Table 2: Total Viable LAB Count of Pasteurised and Probiotificated Orange and Cocktail Juices

Note: Each value represents the mean of three replicate counts.
3.2 Morphological and Biochemical Characteristics of LAB
Table 3 details the morphological and biochemical characteristics of the LAB isolated from Ogi and the kefir starter culture (KSC) when inoculated into tomato juice. The results confirmed that Lactobacillus spp. were the predominant isolates. The LAB isolates from Ogi were small, circular, and smooth, with entire margins and raised elevations. For instance, Lactobacillus casei appeared translucent and brown, while Lactobacillus acidophilus was creamy and opaque. All isolates demonstrated positive Gram reactions and were negative for citrate utilisation, catalase, indole production, and oxidase activity. In addition, they were non-motile, non-spore-forming and fermented glucose.
Similarly, the Lactobacillus acidophilus isolated from KSC in tomato juice was circular with smooth, entire margins, raised elevation, opaque appearance and creamy colour. This isolate also exhibited positive Gram staining, negative reactions to citrate, catalase, indole, and oxidase tests, and was non-motile, non-spore-forming, and a glucose fermenter.
Table 3: Morphological and Biochemical Characteristics of LAB Isolated from Ogi and Kefir Starter Culture

Key: Og = Ogi isolate; Ksc = Kefir starter culture; + = Positive; – = Negative; CC = circular, Et = entire; Rs = raised; Cr = cream; Br = brown; Ts = translucent; Op = opaque.
3.3 Probiotification Effects on Physicochemical Parameters of the Produced Juices
Figures 1 to 4 illustrate the effects of probiotification on various physicochemical parameters of the pasteurised and probiotificated juices. Figure 1 shows that pasteurised juices (orange and cocktail) initially had higher pH values of 3.95 and 3.78, respectively, with a noticeable decline following probiotification. Similarly, Figure 2 demonstrates that the Brix composition (indicative of sugar content) was higher in the pasteurised juices than in the probiotificated juices. Figure 3 reveals an increase in total titratable acidity (TTA) over time; the pasteurised juices exhibited the highest TTA values, although no significant differences were observed between probiotic orange and pasteurised orange juices on the final day. In contrast, Figure 4 indicates that there was no discernible effect of probiotification on the temperature of the samples.
3.4 Nutritional Composition of the Produced Juices
Figure 5 displays the nutritional composition—including moisture content, ash, protein, fat, nitrogen-free extract (NFE) and vitamin C—of the produced juices (orange, cocktail, probiotic orange, and probiotic cocktail). The pasteurised cocktail juice exhibited the highest moisture content (93.65 g/100 g), followed by pasteurised orange juice (91.89 g/100 g), probiotic cocktail juice (91.55 g/100 g) and probiotic orange juice (90.95 g/100 g). The highest ash content (0.53 g/100 g) was recorded in the probiotic orange juice, whereas the probiotic cocktail juice had the lowest ash content (0.42 g/100 g). Protein content was markedly higher in the probiotic orange juice (3.57 g/100 g) compared with pasteurised orange juice (0.58 g/100 g). The fat content in probiotic orange juice (0.63 g/100 g) was slightly above that of the pasteurised orange juice (0.58 g/100 g), and the NFE content was 2.73 g/100 g. Vitamin C content peaked in pasteurised orange juice (29.63 g/100 g), followed by pasteurised cocktail juice (20.11 g/100 g), probiotic orange juice (26.12 g/100 g) and probiotic cocktail juice (16.54 g/100 g).
3.5 PCR and Molecular Characterisation
The results from agarose gel electrophoresis for the isolated samples are presented in Plate 4. Genomic DNA of each probiotic isolate was extracted using a Presto gDNA kit, followed by gel electrophoresis, PCR amplification and subsequent confirmation. The lanes in Plate 4 correspond to the following: “A –” indicates the Solar Bio DNA 1 kb Ladder, “O4 –” corresponds to Lactobacillus acidophilus sF16s, “O5 –” corresponds to Lactobacillus casei DSM 20011, while “S4 –” and “S5 –” correspond to additional replicates of Lactobacillus acidophilus sF16s.
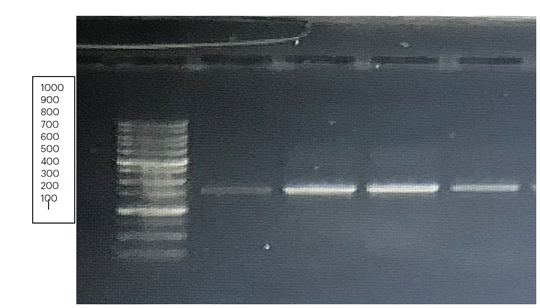
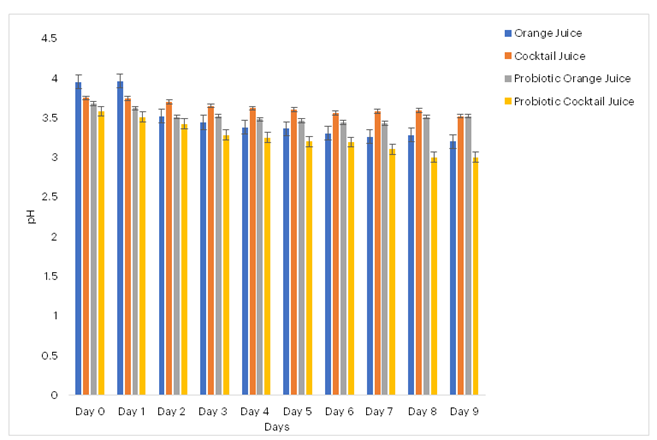
Figure 1: Effects of Probiotification on pH composition on the produced juices
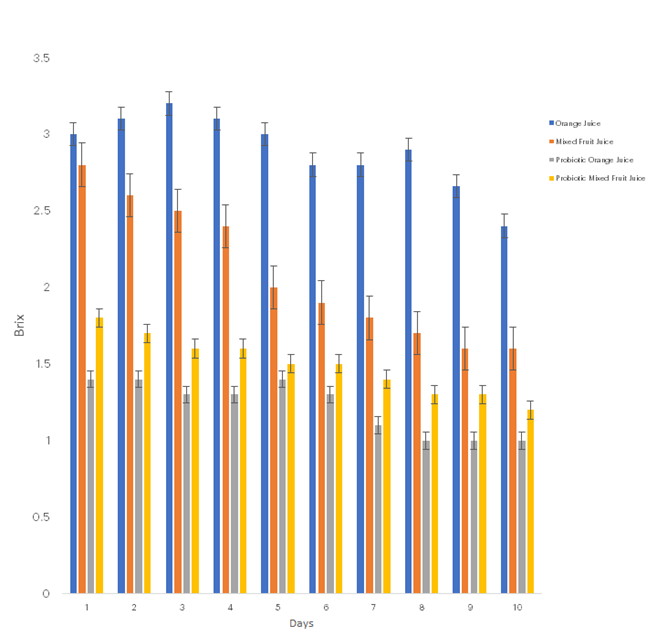
Figure 2: Effects of Probiotification on Brix composition on the produced juices

Figure 3: Effects of Probiotification on Total Titratable Acid (TTA) composition on the produced juices

Figure 4: Effects of Probiotification on Temperature composition on the produced juices

Figure 5: The Effect of Probiotification on the Nutritional Composition of the Juices
The nutritional composition of the juices was evaluated by measuring moisture content, ash, protein, fat, nitrogen-free extract (NFE) and vitamin C levels. The results are summarised as follows:
3.5.1 Moisture Content
The pasteurised cocktail juice exhibited the highest moisture content (93.65 g/100 g), followed by pasteurised orange juice (91.89 g/100 g), probiotic cocktail juice (91.55 g/100 g) and probiotic orange juice (90.95 g/100 g).
3.5.2 Ash Content
The probiotic orange juice showed the highest ash content (0.53 g/100 g), whereas the probiotic cocktail juice recorded the lowest ash content (0.42 g/100 g).
3.5.3 Protein Composition
A markedly higher protein level was observed in probiotic orange juice (3.57 g/100 g) compared with pasteurised orange juice (0.58 g/100 g).
3.5.4 Fat Content and NFE
Probiotic orange juice also had a slightly higher fat content (0.63 g/100 g) relative to pasteurised orange juice (0.58 g/100 g), and the NFE was recorded at 2.73 g/100 g.
3.5.5 Vitamin C Content
Vitamin C levels were highest in pasteurised orange juice (29.63 g/100 g), followed by probiotic orange juice (26.12 g/100 g), pasteurised cocktail juice (20.11 g/100 g) and probiotic cocktail juice (16.54 g/100 g).
3.6 Shelf-Life Study of the Produced Probiotic Juices
The shelf-life of the probiotificated juices was assessed by monitoring key physicochemical parameters—namely pH, Brix (total soluble solids), total titratable acidity (TTA) and temperature—after the addition of natural preservatives.
3.6.1 pH Measurements
Figure 6 shows that the probiotic orange juice had an initial pH of 3.69, whereas the probiotic cocktail juice with lemon essential oil started at 3.43. The chemically preserved probiotic juices maintained a stable pH range of 3.5 to 3.4 over time. In contrast, the unpreserved probiotic juices, particularly the probiotic cocktail juice, experienced a decline in pH from 3.43 to 3.18, while the probiotic orange juice declined from 3.63 on day 1 to 3.42 by the end of the observation period.
3.6.2 Brix (Total Soluble Solids)
As shown in Figure 7, unpreserved probiotic orange juice began with a Brix value of approximately 3.0, increased to about 3.2 and then steadily declined to 2.7, indicating a gradual reduction in sugar content likely due to conversion into organic acids.
3.6.3 Total Titratable Acidity (TTA)
Figure 8 demonstrates that the TTA increased over time, with the naturally preserved probiotic cocktail juice using lime essential oil maintaining the highest TTA value until the final day of the experiment. This increase reflects the ongoing production of organic acids by the LAB.
3.6.4 Temperature
Figure 9 displays that the temperature of both preserved (natural and chemical) and unpreserved probiotic orange and cocktail juices remained relatively unchanged throughout the study, reinforcing that preservation methods did not significantly affect the thermal stability of the juices.
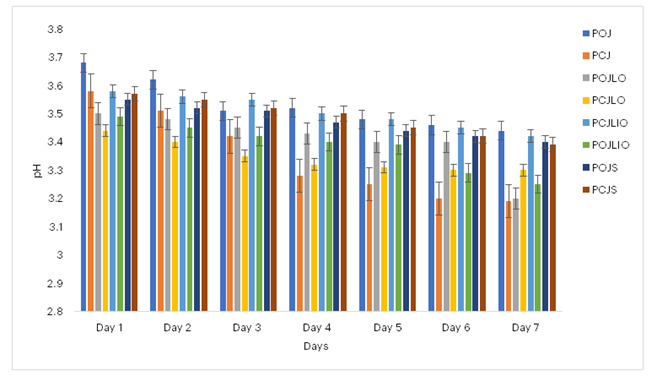
Figure 6: Effects of Preservation on the pH of Probiotificated Juices
Key: POJ: Probiotic Orange Juice; PCJ: Probiotic Cocktail Juice; POJLO: 1% Lemon essential oil Probiotic Orange Juice; PCJLO: 1% Lemon essential oil Probiotic Cocktail Juice; POJLIO: 1% Lime essential oil Probiotic Orange Juice; PCJLIO: 1% Lime essential oil Probiotic Cocktail Juice; POJS: 1% Sodium sulphite Probiotic Orange Juice; PCJS: 1% Sodium sulphite Probiotic Cocktail Juice.
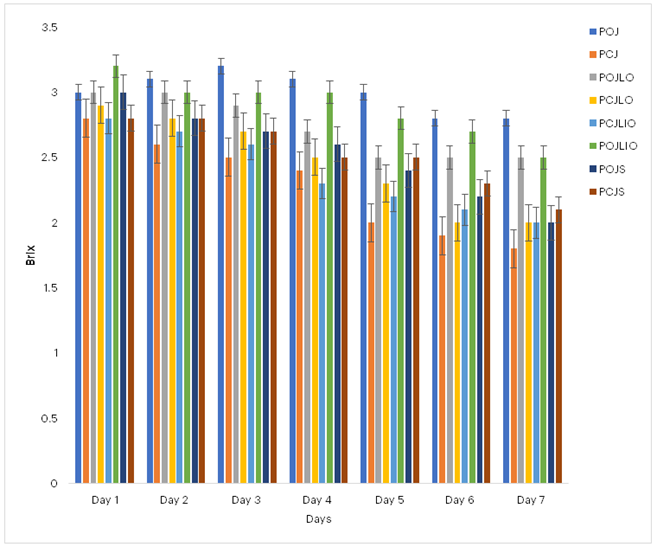
Figure 7: Effects of Preservation on the Brix of Probiotificated Juices Key: POJ: Probiotic Orange Juice; PCJ: Probiotic Cocktail Juice; POJLO: 1% Lemon essential oil Probiotic Orange Juice; PCJLO: 1% Lemon essential oil Probiotic Cocktail Juice; POJLIO: 1% Lime essential oil Probiotic Orange Juice; PCJLIO: 1% Lime essential oil Probiotic Cocktail Juice; POJS: 1% Sodium sulphite Probiotic Orange Juice; PCJS: 1% Sodium sulphite Probiotic Cocktail Juice
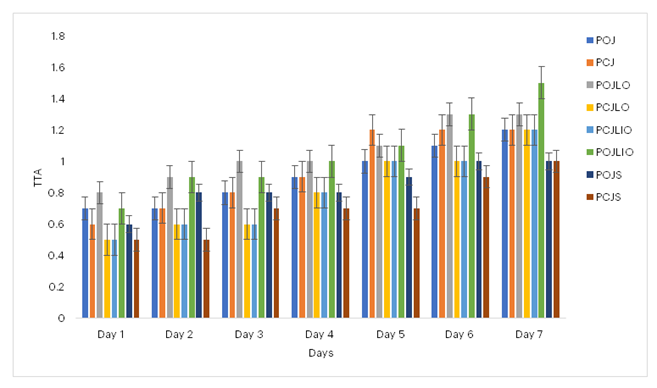
Figure 8: Effects of Preservation on the Total Titratable Acidity (TTA) of Probiotificated Juices
Key: POJ: Probiotic Orange Juice; PCJ: Probiotic Cocktail Juice; POJLO: 1% Lemon essential oil Probiotic Orange Juice; PCJLO: 1% Lemon essential oil Probiotic Cocktail Juice; POJLIO: 1% Lime essential oil Probiotic Orange Juice; PCJLIO: 1% Lime essential oil Probiotic Cocktail Juice; POJS: 1% Sodium sulphite Probiotic Orange Juice; PCJS: 1% Sodium sulphite Probiotic Cocktail Juice
Figure 9: Effects of Preservation on the Temperature of Probiotificated Juices
Key: POJ: Probiotic Orange Juice; PCJ: Probiotic Cocktail Juice; POJLO: 1% Lemon essential oil Probiotic Orange Juice; PCJLO: 1% Lemon essential oil Probiotic Cocktail Juice; POJLIO: 1% Lime essential oil Probiotic Orange Juice; PCJLIO: 1% Lime essential oil Probiotic Cocktail Juice; POJS: 1% Sodium sulphite Probiotic Orange Juice; PCJS: 1% Sodium sulphite Probiotic Cocktail Juice
4. Discussion
The present study revealed that the moisture content of pasteurised juices was higher than that of the probiotificated juices, a finding that is in agreement with Rafiq et al. (2016), who reported a reduction in moisture content following the addition of probiotic microorganisms. Conversely, certain nutritional parameters, such as protein and fat content, were enhanced in the probiotic juices compared with their pasteurised counterparts. This increase may be attributed to the presence and metabolic activity of the probiotic organisms and their secreted metabolites.
The physicochemical properties of both unprobiotificated and probiotificated juices were thoroughly examined. Notably, the presence of lactic acid bacteria (LAB) was associated with a significant reduction in pH and a corresponding increase in acidity over time, primarily due to lactic acid production. As illustrated in Figure 6, the increased acidity—reflected by the decreasing pH—suggests enhanced digestibility of carbohydrates and proteins, consistent with the findings of Sindhu and Khetarpaul (2001). Moreover, the observed reduction in Brix values, as the day progressed, can be explained by the conversion of sugars into organic acids, a relationship that has also been documented by Hossain et al. (2019). These interdependent parameters underscore the dynamic changes occurring during fermentation, where a decrease in pH is concomitant with a decline in Brix and a rise in total titratable acidity (TTA), as reported by both Rafiq et al. (2019) and Hossain et al. (2019).
The shelf-life study further demonstrated that the addition of natural preservatives, such as lemon and lime essential oils, as well as chemical preservatives like sodium sulphite, helped to stabilise the pH over time by slowing nutrient utilisation by LAB. For example, chemically preserved probiotic juices maintained a pH range of 3.5 to 3.4 throughout the observation period, whereas the unpreserved probiotic cocktail juice showed a more pronounced decline from 3.43 to 3.18. This suggests that both natural and chemical preservatives can effectively extend the shelf-life of probiotic juices by moderating acid production. These results are in line with those of Ramachandran et al. (2017) and Chauhan et al. (2015), who reported similar trends in the maintenance of physicochemical stability in juices treated with natural preservatives. Furthermore, the low pH observed in the samples not only indicates effective lactic acid production but also contributes to the bacteriostatic and bacteriocidal effects against foodborne pathogens, as described by Tomaino et al. (2005) and supported by the USFDA (2011) guidelines regarding GRAS (generally recognised as safe) substances. The antimicrobial properties of the essential oils, in combination with the metabolic by-products of LAB, likely play a key role in inhibiting the growth of pathogenic and spoilage microorganisms.
5. Conclusion
The probiotic effects of lactic acid-producing bacteria were found to be significantly pronounced on both the nutritional and chemical compositions of the orange and cocktail juices. The fortification of these juices with selected LAB resulted in enhanced protein and fat content, as well as increased acidity and reduced pH due to lactic acid production. Moreover, the use of natural preservatives, in conjunction with chemical agents, effectively extended the shelf-life of the juices, thereby preserving their nutritional quality and safety. These findings support the potential of biofortification of fermented foods—particularly fruit juices with desirable starter cultures as a viable strategy for improving food quality and safety. Future studies should further optimise fermentation parameters and explore additional fruit matrices to fully harness the benefits of probiotification in non-dairy food systems.
Acknowledgement
The authors acknowledge the support of their affiliated institutions and the technical assistance provided by the laboratory staff.
Funding
No financial support was received for this study.
Conflict of Interest
The authors declare that they have no competing interests.
References
Al‐Jabri, N. N., & Hossain, M. A. (2018). Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. Journal of King Saud University-Science, 30(1), 14–20.
Adelekan, A. O., & Oyewole, O. B. (2010). Production of Ogi from germinated sorghum supplemented with soybeans. African Journal of Biotechnology, 9(42), 7114–7121.
Anadon, A., Irma, A., Martínez-Larrañaga, M., & María-Aránzazu, M. (2021). Nutraceuticals: Efficacy, safety and toxicity. In R. C. Gupta, R. Lall, & A. Srivastava (Eds.), Probiotics: Safety and toxicity considerations (Vol. 2, pp. 1081–1105). Academic Press.
Bagci, U., & Temiz, A. (2011). Microbiological quality of fresh-squeezed orange juice and efficacy of fruit surface decontamination methods in microbiological quality. Journal of Food Protection, 74(8), 1238–1244.
Carlier, J. D., d’Eeckenbrugge, G. C., & Leitão, J. M. (2014). Pineapple: Genome mapping and molecular breeding in plants, fruits and nuts. In Springer-Verlag Berlin Heidelberg (pp. 331–342).
Chaitow, L., & Trenev, N. (2002). Probiotics. Retrieved from http://www.natren.com
Chauhan, O. P., Dheer-Singh, S. M., & Balyan, D. K. (2002). Studies on preservation of sugarcane juice. International Journal of Food Properties, 5(1), 217–229.
Cheesbrough, M. (2022). Biochemical tests to identify bacteria. In Laboratory Practice in Tropical Countries (2nd ed., pp. 63–70). Tropical Health Technology.
Etebu, E., & Nwazouma, A. B. (2011). A review on sweet orange (Citrus sinensis): Health, diseases, and management. American Journal of Research Communication, 2, 33–70.
Farag, R. S., Daw, Z. Y., & Abo-Raya, S. H. (2006). Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. Journal of Food Science, 54(6), 74–76.
Fazeli, M. R., Bahmania, S., Jamalifara, H., & Samadi, N. (2011). Effect of probiotification on antioxidant and antibacterial activities of pomegranate juices from sour and sweet cultivars. Natural Product Research, 25, 288–297.
Gasbarrini, G., Bonvicini, F., & Gramenzi, A. (2016). Probiotics history. Journal of Clinical Gastroenterology, 50, S116–S119.
Greppi, A., Rantsiou, K., Padonou, W., Hounhouigan, J., Jespersen, L., Jakobsen, M., & Cocolin, L. (2013). Determination of yeast diversity in Ogi, Mawè, Gowé, and Tchoukoutou by using culture-dependent and independent methods. International Journal of Food Microbiology, 165(2), 84–88.
Hidayat, H., Haryadi, W., Matsjeh, S., & Raharjo, T. J. (2019). Molecular identification 16S rRNA gene of active proteolytic lactic acid bacteria (LAB) isolated from kelengkeng (Dimocarpus longan) fruit. Biodiversitas Journal of Biological Diversity, 20(8), 2222–2228.
Hill, C., Guarner, F., & Reid, G. (2014). The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11, 506–514.
Hossain, M. A., Hoque, M. M., Kabir, M. H., & Yasin, M. (2019). Probiotification of mango juice by lactic acid bacteria and quality assessment at refrigerated storage condition. Journal of Engineering Research, Innovation and Education, 1, 2–10.
Jaya, P. B., & Dubey, N. K. (2011). Evaluation of chemically characterised essential oils of Coleus aromaticus, Hyptis suaveolens and Ageratum conyzoides against storage fungi and aflatoxin contamination of food commodities. International Journal of Food Science and Technology, 46(4), 754–760.
Olaniran, A. F., & Abiose, S. H. (2019). Nutritional evaluation of enhanced unsieved Ogi paste with garlic and ginger. Preventive Nutrition and Food Science, 24(3), 348.
Olaniran, A. F., Abiose, S. H., Adeniran, H. A., Gbadamosi, S. O., & Iranloye, Y. M. (2020). Production of a cereal-based product (Ogi): Influence of co-fermentation with powdered garlic and ginger on the microbiome. Agrosearch, 20(1), 81–93.
Omemu, A. M. (2011). Fermentation dynamics during production of Ogi, a Nigerian fermented cereal porridge. Report and Opinion, 3(4), 8–17.
Otles, S., & Cagindi, O. (2003). Kefir: A probiotic dairy—Composition, nutritional and therapeutic aspects. Pakistan Journal of Nutrition, 2(2), 54–59.
Prakash, B., Singh, P., Kedia, A., & Dubey, N. K. (2012). Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Research International, 49(1), 201–208.
Rafiq, S., Sharma, V., Nazir, A., Rashid, R., & Sofi, S. A. (2016). Development of probiotic carrot juice. Journal of Nutrition and Food Sciences, 6(2), 2–4.
Rafiq, K., Shaheen, N., & Shah, M. H. (2019). Evaluation of antioxidant activities and essential/toxic metal levels and their health risk assessment in citrus fruits from Pakistan. Environmental Monitoring and Assessment, 191, 1–14.
Ramachandran, C., Rani, S. D., Lavanya, K., Nivetha, S., & Usha, A. (2017). Optimisation of shelf stability of sugarcane juice with natural preservatives. Journal of Food Processing and Preservation, 41(1), 128–168.
Sanders, M. E., Merenstein, D., Merrifield, C. A., & Hutkins, R. (2018). Probiotics for human use. British Nutrition Foundation Nutrition Bulletin, 43, 212–225.
Sindhu, S. C., & Khetarpaul, N. (2001). Probiotic fermentation of indigenous food mixture: Effect on antinutrients and digestibility of starch and protein. Journal of Food Composition and Analysis, 14(6), 601–609.
Soliman, K. M., & Badeaa, R. I. (2002). Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Journal of Food Chemistry and Toxicology, 40(2), 1669–1675.
Speranza, B., & Corbo, M. R. (2010). Essential oils for preserving perishable foods: Possibilities and limitations. In A. Bevilacqua, M. R. Corbo, & M. Sinigaglia (Eds.), Application of alternative food-preservation technologies to enhance food-safety and stability (pp. 35–57). Bentham Publisher.
Tomaino, A., Cimino, F., Zimbalatti, V., Venuti, V., Sulfaro, V., & De Pasquale, A. (2005). Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Journal of Food Chemistry, 89(2), 549–554.
USFDA. (2011). Food additive status list. U.S. Food and Drug Administration. Retrieved from http://www.cfsan.fda.gov/dms/rdb/opa-appa.html
Zheng, J., Wittouck, S., Salvetti, E., Franz, M. A. P., Harris, M. B., Mattarelli, P., O’Toole, P. W., Pot, B., Vandamme, P., Walter, J., & Watanabe, K. (2020). A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology, 70(4), 2782–2850.
About this Article
Cite this Article
APA
Gabriel-Ajobiewe R.A.O., Ademoyegun S.O., Adeleke B.S. & Bankefa E.O.(2025). Effect of Lactic Acid Bacteria as a Tool for Probiotification on the Composition of Orange and Cocktail Juices. In Akinyele B.J., Kayode R. & Akinsemolu A.A. (Eds.), Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye. SustainE
Chicago
Gabriel-Ajobiewe R.A.O., Ademoyegun S.O., Adeleke B.S. and Bankefa E.O. 2025. “Effect of Lactic Acid Bacteria as a Tool for Probiotification on the Composition of Orange and Cocktail Juices” In Microbes, Mentorship, and Beyond: A Festschrift in Honour of Professor F.A. Akinyosoye, edited by Akinyele B.J., Kayode R. and Akinsemolu A.A., SustainE.
Received
10 November 2024
Accepted
20 January 2025
Published
4 February 2025
Corresponding Author Email: kennyeniola@gmail.com
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.