Ovinuchi Ejiohuo 1
1Doctoral School, Poznan University of Medical Sciences, Poznan, Poland
*Corresponding Author Email: ejiohuo@gmail.com
Highlights
- Biodegradable, renewable PLA offers a sustainable alternative to traditional plastics.
- Microbial enzymes enhance PLA properties, providing eco-friendly plastic degradation.
- Blending PLA with TPU addresses fragility, creating durable, flexible materials.
- Proteinase K, Savinase, Alcalase optimize PLA degradation processes effectively.
- Ongoing research on microbial enzymes is crucial for advancing PLA recycling methods.
Graphical Abstract
Abstract
Polylactic acid (PLA) has emerged as a desirable bioplastic due to its production from renewable materials and biodegradability. However, its low toughness and fragility limit its applications, prompting the blending of PLA with other biopolymers to enhance its properties. As the global demand for bioplastics increases, efficient processes for PLA degradation are needed to match the high rate of plastic production. Chemical, microbial, and enzymatic processing are the major methods of PLA degradation, with enzymatic processing being environmentally friendly and sustainable. recyclable products like lactic acid can also be recovered. This creates a need to determine suitable enzymes that can hydrolyze polylactic acid. PLA exhibits high resistance to direct microbial degradation, making microbial enzymatic processing a more attractive alternative. Microbial enzymes, including proteases, lipases, esterases, and cutinases, have shown potential for PLA degradation. Nevertheless, current research on the enzymatic degradation of PLA needs comprehensive studies on optimal processes, conditions, and influencing factors. This review aims to address this gap by examining microbial enzymes and their processes for the enzymatic degradation of PLA. Findings indicate that microbial enzymes like proteinase K, Savinase, and Alcalase show promise for efficient PLA degradation under optimized conditions. Further research should, therefore, focus on exploring these enzymes further, refining enzymatic degradation processes, exploring genetic modifications of these enzymes, and developing sustainable recycling methods to advance bioplastics like PLA and address plastic pollution challenges effectively.
Keywords: Polylactic acid; enzymatic degradation; microbial enzymes; optimization; bioplastic; biodegradability; plastic pollution; bioremediation
1. Introduction
Plastic pollution is now a global threat to human and environmental health, with more than 6.3 billion tons of plastic waste produced as of 2019 (Mazhandu & Muzenda, 2019) and is predicted to reach 53 million metric tons by 2030. In 2016, 11% of the plastic waste was in the marine environment (Borrelle et al., 2020). According to Thushari et al., 2020, microplastics have both ecological; toxicological effects (chemical pollution and cosmetic waste), starvation, the emergence of invasive species, and socio-economical; affecting tourism, fishing, shipping, and human health. A recent study by Leslie et al. (2022) found polyethylene terephthalate (PET), polyethylene, and polystyrene microplastics in human blood with exposure able to cause damaging effects on human cells. Another study by Yuan et al. (2022) elucidated the role of polystyrene microplastic in antibiotic resistance genes (ARGs) transfer. This shows the extent to which non-biodegradable plastics are adversely impacting global health. The importance of plastics, their global demand, and the high cost of recycling single-use plastics have therefore necessitated a shift towards an environmentally friendly plastic alternative that is biodegradable and bio-assimilable.
Bioplastics are biodegradable and bio-based polymer plastics made from renewable resources rather than petrochemical raw materials. According to Endres (2017), they are either made from renewable materials, biodegradable, or both. They, therefore, can be degradable petrochemical-based bioplastics, degradable bio-based bioplastics, and non-degradable bio-based bioplastics. This distinction is important because biodegradable plastics can also be made from petrochemical raw materials (Endres, 2017), such as polybutyrate adipate terephthalate (PBAT) used in the production of bioplastics (Muthusamy & Pramasivam, 2019). Bioplastics can be categorized into three types based on their origin: bioplastics obtained from biomass (PLA), bioplastics obtained from vegetable oils, and bioplastics obtained from polyhydroxyalkanoates (PHA) (Endres, 2017). Bioplastics contribute to climate change mitigation and sustainable development by reducing carbon dioxide emissions (Spierling et al., 2018).
Polylactic acid (PLA) has become the desired bioplastic for various industrial applications since it can be produced from affordable renewable materials (Zaaba & Jaafar, 2020). PLA also degrades easily, but since it has low toughness, it is usually combined in varying ratios with other biopolymers to create plastic blends with enhanced properties (Ferri et al., 2020). As global advocacy for bioplastics increases due to its impact on climate change mitigation and adaptation, there is, therefore, a need for efficient processes for PLA degradation that can match the high rate of plastic production. PLA is a bio-based polymer and a biodegradable bioplastic produced from renewable sources. It is responsible for over 39% of the demand for lactic acid (Teixeira et al., 2021). A widely used process in synthesizing PLA is condensation and polymerization of lactic acid (Sikder et al., 2019). Polylactic acid is highly stable and rigid and has increased transparency and thermoplasticity compared to other plastics (Muthusamy & Pramasivam, 2019). It uses 55% less energy, which could be reduced by 10% in the future (Teixeira et al., 2021). PLA is already widely used in biomedicine like in the production of bio-compatible medical devices (Jiménez, 2019), such as reusable N95 face masks against Covid-19 (NatureWorks, 2020), artificial heart valves, sutures in tissue engineering, and drug delivery systems (Mann et al., 2021). These devices made from PLA have been shown not to damage tissues or cause cancers. This also means they can degrade inside the human body without needing removal. The packaging, textile, agriculture, and automobile industries (Qi et al., 2017) are industries where PLA is extensively used. Attention is being given to the use of PLA in the packaging industry since this sector has the greatest demand for plastics (Jiménez, 2019). PLA has mechanical properties such as low turbidity, high tensile strength, and tear resistance that can be compared to petrochemical polymers, making it a better alternative for plastic production (Jiménez, 2019; Rivero et al., 2017). Table 1 below shows the properties of the different types of polylactic acid.
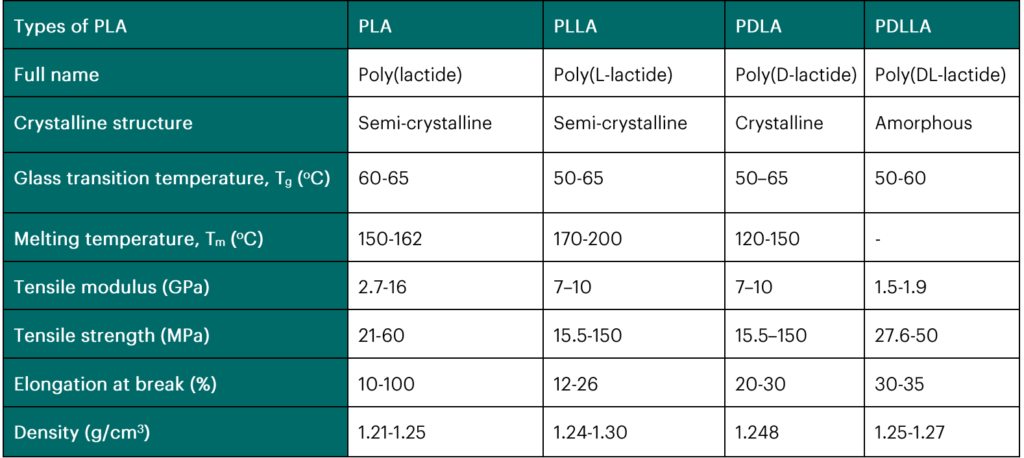
Table 1: Properties of PLA, PLLA, PDLA, and PDLLA (Zaaba & Jaafar, 2020, Farah et al., 2016; Shuaib et al., 2019; Munim & Raza, 2019).
Current research involving the enzymatic degradation of PLA has proven successful (Sourkouni et al., 2023). A comprehensive bioprocess is however needed for examining potential enzymes and process conditions suitable for the degradation of PLA will provide a validated process useful for further research and identify suitable enzymes that can further be engineered to be more effective. To optimize the enzymatic degradation process of PLA, we need to continuously review the microbial enzymes involved to understand the processing parameters and conditions. This will give us an insight into the best optimization strategies. The mechanism of enzyme-substrate interaction during the PLA degradation is also not well known (Mohanan et al., 2020) hence, this review makes an effort to focus on the microbial enzymes, especially commercially available enzymes, to shed more light on them. This will highlight the effectiveness of microbial enzymes, particularly proteases, in degrading PLA and identify areas for further research, including optimized experimental designs, genetic modification, and investigation of biopolymer blends. The ultimate goal is to contribute to developing efficient and sustainable methods for the degradation and recycling of PLA, addressing the challenges of plastic pollution. Figure 1 below gives a summary of this process.

2. Blend of Polylactic Acid and Thermoplastic Polyurethane
While PLA exhibits desirable properties, such as being biodegradable and having high tensile strength, it is easily degraded, making it fragile and limiting how extensively it can be applied for varying uses. On the other hand, thermoplastic polyurethane (TPU) properties can solve PLA’s problem with toughness. At high temperatures, TPU becomes soft with low pressure and exhibits a tensile strength even higher than that of rubber (Szefer et al., 2019). Its physical and chemical properties make it highly elastic, flexible, and resistant to wear and tear, hence its applicability in biomedicine (Princi, 2019; Xiang et al., 2019). Thermoplastic polyurethane has the following properties: high hardness, difficult to burn, rough, strong, high abrasion resistance, softens and deforms above 250oC, unable to hold a large amount of weight and has a specific gravity of 1.2 (Kopal et al., 2019; Allami et al., 2021). Thermoplastic polyurethane is not traditionally biodegradable, but hydrolytic degradation can occur under a vacuum (Yuan et al., 2019). Degradation under this condition poses a challenge, for example, for biomedical implants that are expected to stay long in the human body (Herzberger et al., 2019).
Research is blending PLA with other biopolymers like TPU to produce long-lasting materials such as laptops, computer parts, mobile devices, and automobile parts (Zhou et al., 2015). A study describes a strategy for producing 4D materials that used a blend of polylactic acid, thermoplastic polyurethane, and carbon nanotube (PLA/TPU/CNT) (Dong et al., 2021). Nordin et al. (2019) observed a significant increase in elongation at break when PLA was blended with TPU to prepare polymer blends to develop conductive polymers. Another study found that PLA/TPU blends of 80/20 and 60/40 had the most desirable annealing effect on the shape recovery ratio (Lai et al., 2016). In another study, the PLA/TPU bend 80/20 had a higher recovery ratio than the 50/50 blend compared to Lee et al. (2011) (Doğan et al., 2017). Enzymatic degradation of PLA within PLA/TPU blends is a valuable tool for recycling and disposal as it offers a sustainable and resource-efficient approach to separate and recycle PLA while preserving the properties of TPU. The enzymes can selectively target the PLA component. In the study by Doğan et al., 2017, the enzymatic degradation rate of PLA was faster than that of TPU . In the study by Liu et al. 2000, Proteinase K targeted only the amorphous parts of PLLA rather than the crystalline parts of PLLA and PCL. TPU is highly crystalline and generally resistant to enzymatic degradation. Enzymes like Proteinase K do not have the same specificity for TPU as they do for PLA (Rodolfo et al., 2022). This process aligns with circular economy principles, reduces environmental impact, and encourages responsible waste management practices in the context of plastic recycling and can be applied to the degradation of PLA in other polymer blends.
3. Enzymatic Degradation of Polylactic Acid
An optimal or ideal degradation process of PLA manages the end-of-life of PLA and ensures environmental sustainability. Under natural environmental conditions, PLA materials can undergo degradation that might cause an irreversible change and result in the loss of useful properties (Zaaba & Jaafar, 2020). Under suitable conditions, however, PLA breaks down into water and carbon dioxide or lactic acid (Da Silva et al., 2018), an important recyclable product. Enzymatic degradation of PLA is a two-step process involving first the adsorption of the enzyme on the surface of PLA and, secondly, the hydrolysis of the PLA ester bonds (Sukkhum & Kitpreechavanich, 2011; Tokiwa & Calabia, 2006). It generally requires less energy compared to mechanical separation techniques. According to the review by Costa et al. (2023), PLA energy requirement can be as low as 7.4 MJ kg-1.
Enzymatic activity in the degradation of PLA is low in acidic conditions, especially when compared with enzyme activity in alkaline conditions. This is because hydrolysis of the ester bonds in PLA is faster and more efficient in alkaline conditions than in acidic conditions (Koterwa et al., 2022). Gravimetric analysis can confirm the percentage weight loss following HPLC analysis. The weight loss will result from the release of soluble monomers and oligomers formed during the hydrolysis of the PLA (Tsuji & Miyauchi, 2001). In the study by Tsuji & Miyauchi, 2001, positive enzymatic hydrolysis rate (R(EH)) of 1.75 microg/(mm(2).h) values when the polymer was dried under reduced pressure for 14 days, and another study by Lee et al. (2011), dried PLA nonwoven samples in an oven at 105oC for 90 minutes. In a similar study by Czarnecka-komorowska et al. (2021), samples of PLA blends were weighted at intervals of 30 minutes to observe the mass stabilization. This is a good approach, as penetrating water molecules can cause a physical or chemical change to polymer materials. They dried the composite material again and observed a slight weight loss of 0.08% in the PLA blend to obtain a more precise result.
Applicable methods of PLA degradation majorly involve chemical, microbial, and enzymatic processing (Zaaba & Jaafar, 2020). While chemical processing causes pollution (Reddy et al., 2008), microbial and enzymatic processing is environmentally friendly (Xu et al., 2022). It ensures the sustainable recycling of useful components (Sukkhum & Kitpreechavanich, 2011). Microbial and enzymatic processing is of greater importance because they form water and carbon dioxide (Zaaba & Jaafar, 2020). PLA, however, is highly resistant to microbial degradation (Teixeira et al., 2021), making enzymatic processing a potentially more efficient and attractive alternative. With enzymatic processing, advantages include high specificity of the enzyme, mild reaction conditions, and no substrate loss due to chemical modifications (Teixeira et al., 2021; Huang et al., 2020; Myburgh et al., 2023; Singhvi et al., 2019). In the study by Li et al. 2008, PLA depolymerase enzyme isolated from Amycolatopsis sp. was able to degrade PLA of a high molecular weight. The main difference between microbial and enzymatic processing is that the microorganism acts on the plastic material by releasing its plastic-degrading enzyme or enzymes in the former. It is, in fact, the enzymes that do the work and not the microorganism. The study by Bubpachat et al. (2018) focused on the degradation of PLA by PLA-degrading bacterium isolates that produced the enzymes and wasn’t concerned with the isolation and characterization of the enzymes. Although the review by Mohanan et al. (2020), gave an overview of microbial enzymes involved in the degradation of plastics, it did not discuss the action of these enzymes on PLA (Mohanan et al., 2020). Enzymatic processing, on the other hand, involves the direct action of the enzymes on the plastic material; hence, it is considered more specific in action. In enzymatic processing, the enzymes used could be synthetic or of microbial or plant origin. The review by Xu et al. (2022) discussed PLA’s microbial and enzymatic degradation and highlighted the enzymes involved. It included the factors influencing PLA degradation, such as recycling, upcycling, physiochemical conditions, geographical location, and climate change. The main enzymes used in the enzymatic degradation of PLA are of microbial origin. Proteases that are hydrolytic enzymes have been identified to degrade PLA efficiently (Hegyesi et al., 2019). Other enzymes, such as lipase, esterase (Akutsu-Shigeno, et al., 2003), and cutinase (Masaki, et al., 2005), have also been linked to PLA degradation.
The reality of PLA degradation is, however, complex. Its production also requires significant energy and resources, with 54.1 MJ kg−1 required for producing PLA, however lower than that needed for producing petrochemical polymers (Costa et al., 2023). Regarding resource strain, for instance, the increased use of starch in lactic acid fermentation makes starch less available for human use (Sun, et al., 2022). Without proper disposal infrastructure and recycling practices, PLA can contribute to plastic pollution by persisting in the environment, harming ecosystems when consumed by living systems, and hindering efforts to mitigate the environmental impact of plastic waste (Rezvani Ghomi et al., 2021). A comprehensive approach to waste management, education, and sustainable alternatives is needed to address these challenges.
4. Overview of key microbial enzymes involved in PLA degradation
The first study that employed the enzymatic degradation of PLA used proteinase K from Tritirachium album and was published in 1981 by David F. Williams (Williams, 1981). Previous studies have shown that the most exemplary and efficient enzyme used in PLA degradation is proteinase K. Enzymes that degrade PLA are majorly proteases (especially serine proteases) of microbial origin (Zaaba & Jaafar, 2020; Hanphakphoom et al., 2014).
The study by Hegyesi et al. (2019) describes the enzymatic degradation of PLA catalyzed by lipase from Candida rugosa and proteinase K from Tritirachium album. The results show that the lipase could not degrade PLA effectively. However, proteinase K was efficient in degrading PLA. Degradation occurred in three steps:
(1) the adsorption of the enzyme on the surface of the substrate,
(2) the enzymatic degradation of the polymer, and
(3) the denaturation of the enzyme.
The degradation rate was highest initially but decreased over time due to denaturation of the enzyme. The accumulation of lactic acid in the reaction medium decreased pH to almost 4, which subsequently caused the denaturation of the enzyme (the degradation occurred below pH 6.5). The solution’s small ionic strength and the cellulose nanocrystals in the PLA mix positively influenced the degradation of the PLA (Hegyesi et al., 2019). In the study by Donate et al. (2020), the enzymatic degradation of PLA by proteinase K was studied in the context of tissue engineering applications, particularly in the development of composite scaffolds made of PLA, calcium carbonate (CaCO3), and β-tricalcium phosphate (β-TCP). The enzymatic degradation process caused the PLA to break down more rapidly than neat PLA scaffolds, especially during the initial stages of the experiment. After 10 days, it was observed that the release of additive particles contributed to increased porosity, enhanced degradation rate, and changes in the mechanical properties of the scaffolds. Although enzymes usually attack the amorphous part of PLA, degradation of the crystalline part was also observed.
Savinase is also an enzyme capable of degrading PLA. In the study by Oda et al. 2000, 66 commercially available proteases were screened for their ability to degrade PLA. Savinase, produced by Bacillus sp., classified as an alkaline protease, showed the fastest degradation of PLA among the enzymes tested. Its activity was 50% that of proteinase K. The degradation of PLA in this study was influenced by the type of protease, its specific activity, pH conditions (activities of the enzymes were tested at their respective optimal pH values with Savinase having an optimal pH of 8-12), and the potential relationship between the degradation of PLA and keratin. The origin of the enzyme was also an influencing factor. A similar study by Ejiohuo, 2022, highlighted in a conference abstract, is an effective enzymatic degradation of PLA using commercially available microbial enzymes. The results demonstrate that alkaline conditions promote enzyme hydrolysis of PLA, with Proteinase K and Savinase showing the highest enzymatic activity in terms of lactic acid production. Bioreactor experiments further validated the effectiveness of Savinase in degrading PLA and producing lactic acid. Maintaining a constant and optimal pH of 10 enhanced the enzymatic activity and degradation efficiency of Savinase in the bioreactor (92.53% of polylactic acid degraded), highlighting the importance of optimizing pH conditions for efficient enzymatic degradation of PLA. The findings from this study are certainly thought-provoking and agree with the findings from the study by Oda et al. (2000) that found Savinase to be second best to Proteinase K. This means that future optimized studies can further show microbial enzymes such as Savinase to be a suitable commercially available candidate for genetic modification to enhance its enzymatic activity (Lu et al., 2022), as achieved by a study that used Machine Learning to genetically engineer a polyethene terephthalate (PET) hydrolase called FAST-PETase (functional, active, stable, and tolerant PETase). It could hydrolyse PET plastics in a day and do this at varying pH ranges.
Alcalase is also another microbial enzyme that has been shown to degrade PLA efficiently. In the study by Lee et al. (2014), alcalase, with its optimal treatment conditions at pH 9.5 and 60°C, demonstrates the highest biodegrading activity, causing significant changes in PLA nonwovens. The results showed changes in the width and thickness of the PLA nonwovens, alterations in the degree of crystallinity, surface roughening and the formation of cracks on the fiber surface, weight loss in the PLA nonwovens, and reduction in tensile strength, making the material more fragile. The study investigated the effects of enzymatic degradation over time. It found that the duration of degradation played a critical role. Different enzymes had varying effects on PLA over time, leading to changes in the PLA properties. Alcalase degradation was conducted near the Tg of PLA (around 60°C), affecting crystallinity and the overall degradation process.
Generally, proteinase K, Savinase, and Alcalase are the most highly reported microbial enzymes involved in the degradation of PLA. However, there is not much literature on their use. This creates an avenue for further research on them and other microbial and synthetic enzymes that could degrade PLA. These enzymes could be in their commercial or modified form. An ideal degradation process for PLA would involve using microbial enzymes with proven efficiency in degrading PLA, such as proteinase K, Savinase, and Alcalase. It will involve optimizing the conditions for degradation, such as pH and temperature, controlling the duration of the process, and considering the intended application of the degraded PLA and the influence of other factors, such as the presence of other materials.
5. Conclusions and future perspectives
This review highlights the significance of microbial enzymes in PLA degradation. An optimal PLA degradation process should focus on enzymatic degradation, especially under alkaline conditions, and consider factors like pH, temperature, and the influence of other materials. This process offers a sustainable and environmentally friendly approach to managing PLA end-of-life products and contributes to reducing plastic pollution. Given the limited literature on the use of microbial enzymes, there is a need for ongoing research in this area. Therefore, researchers should conduct in-depth studies to further optimize and expand the use of microbial enzymes for PLA degradation. Promoting collaboration between researchers from different fields, including biology, materials science, and engineering, to tackle the subject’s complexity and find innovative solutions for plastic pollution will be a good approach.
It is recommended that researchers and industry should continue exploring microbial enzymes’ potential for PLA degradation. Enzymes like proteinase K, Savinase, and Alcalase have efficiently broken down PLA and should be further investigated. There is a need to conduct systematic and well-optimized experiments to understand better the most effective processes and conditions for microbial enzymatic PLA degradation. This includes investigating optimal pH levels, temperature ranges, and reaction times. We should also consider the impact of environmental factors on enzymatic degradation. For example, cellulose nanocrystals have been shown to influence the degradation of PLA positively. Understanding these factors can lead to more efficient degradation processes. Investigating genetic modification techniques that can enhance the enzymatic activity of microbial enzymes will prove beneficial. This approach could make enzymatic degradation even more efficient. Policymakers should consider supporting research and development efforts focused on bioplastics and environmentally friendly degradation methods. This can include funding for research and incentives for industries to adopt sustainable practices. Industries should explore and implement efficient recycling methods for PLA. Microbial enzymatic degradation can play a significant role in the recycling of PLA products.
Author(s) Summary
Funding
This research received no external funding
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflict of Interest
The author declares no conflict of interest.
References
Akutsu-Shigeno, Y., Teeraphatpornchai, T., Teamtisong, K., Nomura, N., Uchiyama, H., Nakahara, T., & Nakajima-Kambe, T. (2003). Cloning and sequencing of a poly (DL-lactic acid) depolymerase gene from Paenibacillus amylolyticus strain TB-13 and its functional expression in Escherichia coli. Applied and Environmental Microbiology, 69(5), 2498-2504. [Google Scholar][CrossRef]
Allami, T., Alamiery, A., Nassir, M. H., & Kadhum, A. H. (2021). Investigating physio-thermo-mechanical properties of polyurethane and thermoplastics nanocomposite in various applications. Polymers, 13(15), 2467. [Google Scholar] [CrossRef].
Borrelle, S. B., Ringma, J., Law, K. L., Monnahan, C. C., Lebreton, L., McGivern, A., … & Rochman, C. M. (2020). Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science, 369(6510), 1515-1518. [Google Scholar] [Cross Ref].
Bubpachat, T., Sombatsompop, N., & Prapagdee, B. (2018). Isolation and role of polylactic acid-degrading bacteria on degrading enzymes productions and PLA biodegradability at mesophilic conditions. Polymer Degradation and Stability, 152, 75-85. [Google Scholar] [CrossRef].
Costa, A., Encarnação, T., Tavares, R., Todo Bom, T., & Mateus, A. (2023). Bioplastics: Innovation for Green Transition. Polymers, 15(3), 517. [Google Scholar] [CrossRef].
Czarnecka-Komorowska, D., Bryll, K., Kostecka, E., Tomasik, M., Piesowicz, E., & Gawdzińska, K. (2021). The composting of PLA/HNT biodegradable composites as an eco-approach to the sustainability. Bulletin of the Polish Academy of Sciences. Technical Sciences, 69(2). [Google Scholar] [CrossRef].
Da Silva, D., Kaduri, M., Poley, M., Adir, O., Krinsky, N., Shainsky-Roitman, J., & Schroeder, A. (2018). Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chemical Engineering Journal, 340, 9-14. [Google Scholar] [CrossRef].
Doğan, S., Boyacioğlu, S., Kodal, M., Gökçe, Ö., & Özkoç, G. (2017). Thermally induced shape memory behavior, enzymatic degradation and biocompatibility of PLA/TPU blends. Journal of the Mechanical Behavior of Biomedical Materials, 71. [Google Scholar] [CrossRef].
Donate, R., Monzón, M., Alemán-Domínguez, M. E., & Ortega, Z. (2020). Enzymatic degradation study of PLA-based composite scaffolds. Reviews on Advanced Materials Science, 59(1), 170-175. [Google Scholar] [CrossRef].
Dong, K., Panahi-Sarmad, M., Cui, Z., Huang, X., & Xiao, X. (2021). Electro-induced shape memory effect of 4D printed auxetic composite using PLA/TPU/CNT filament embedded synergistically with continuous carbon fiber: A theoretical & experimental analysis. Composites Part B: Engineering, 220, 108994. [Google Scholar] [CrossRef].
Ejiohuo, O. (2022). Abstract book for “Enzyme future-Discovery and Engineering”; DTU Bioengineering symposium, p 47. [Website]
Endres, H. J. (2017). Bioplastics. Biorefineries, 427-468. [Google Scholar] [CrossRef].
Farah, S., Anderson, D. G., & Langer, R. (2016). Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Advanced drug delivery reviews, 107, 367-392. [Google Scholar] [CrossRef].
Ferri, J. M., Garcia-Garcia, D., Rayón, E., Samper, M. D., & Balart, R. (2020). Compatibilization and characterization of polylactide and biopolyethylene binary blends by non-reactive and reactive compatibilization approaches. Polymers, 12(6), 1344. [Google Scholar] [CrossRef].
Hanphakphoom, S., Maneewong, N., Sukkhum, S., Tokuyama, S., & Kitpreechavanich, V. (2014). Characterization of poly (L-lactide)-degrading enzyme produced by thermophilic filamentous bacteria Laceyella sacchari LP175. The Journal of General and Applied Microbiology, 60(1), 13-22. [Google Scholar] [CrossRef].
Hegyesi, N., Zhang, Y., Kohári, A., Polyák, P., Sui, X., & Pukánszky, B. (2019). Enzymatic degradation of PLA/cellulose nanocrystal composites. Industrial Crops and Products, 141, 111799. [Google Scholar] [CrossRef].
Herzberger, J., Sirrine, J. M., Williams, C. B., & Long, T. E. (2019). Polymer design for 3D printing elastomers: recent advances in structure, properties, and printing. Progress in Polymer Science, 97, 101144. [Google Scholar] [CrossRef].
Huang, Q., Hiyama, M., Kabe, T., Kimura, S., & Iwata, T. (2020). Enzymatic self-biodegradation of poly (l-lactic acid) films by embedded heat-treated and immobilized proteinase K. Biomacromolecules, 21(8), 3301-3307. [Google Scholar] [CrossRef].
Jiménez, L., Mena, M. J., Prendiz, J., Salas, L., & Vega-Baudrit, J. (2019). Polylactic acid (PLA) as a bioplastic and its possible applications in the food industry. J Food Sci Nutr, 5(2), 2-6. [Google Scholar] [CrossRef].
Kopal, I., Harničárová, M., Valíček, J., Krmela, J., & Lukáč, O. (2019). Radial basis function neural network-based modeling of the dynamic thermo-mechanical response and damping behavior of thermoplastic elastomer systems. Polymers, 11(6), 1074. [Google Scholar] [CrossRef].
Koterwa, A., Kaczmarzyk, I., Mania, S., Cieslik, M., Tylingo, R., Ossowski, T., … & Ryl, J. (2022). The role of electrolysis and enzymatic hydrolysis treatment in the enhancement of the electrochemical properties of 3D-printed carbon black/poly (lactic acid) structures. Applied Surface Science, 574, 151587. [Google Scholar] [CrossRef].
Lai, S. M., Wu, W. L., & Wang, Y. J. (2016). Annealing effect on the shape memory properties of polylactic acid (PLA)/thermoplastic polyurethane (TPU) bio-based blends. Journal of Polymer Research, 23, 1-13. [Google Scholar] [CrossRef].
Lamoree, M. H. (2022). Discovery and quantification of plastic particle pollution in human blood. Environment international, 163, 107199. [Google Scholar] [CrossRef].
Lee, S. H., & Song, W. S. (2011). Enzymatic hydrolysis of polylactic acid fiber. Applied Biochemistry and Biotechnology, 164, 89-102. [Google Scholar] [CrossRef].
Lee, S. H., Kim, I. Y., & Song, W. S. (2014). Biodegradation of polylactic acid (PLA) fibers using different enzymes. Macromolecular Research, 22, 657-663. [Google Scholar] [CrossRef].
Leslie, H. A., Van Velzen, M. J., Brandsma, S. H., Vethaak, A. D., Garcia-Vallejo, J. J., & Li, F., Wang, S., Liu, W., & Chen, G. (2008). Purification and characterization of poly (L-lactic acid)-degrading enzymes from Amycolatopsis orientalis ssp. orientalis. FEMS microbiology letters, 282(1), 52-58. [Google Scholar] [CrossRef].
Li, F., Wang, S., Liu, W., & Chen, G. (2008). Purification and characterization of poly (L-lactic acid)-degrading enzymes from Amycolatopsis orientalis ssp. orientalis. FEMS microbiology letters, 282(1), 52-58. [Google Scholar] [CrossRef]
Liu, L., Li, S., Garreau, H., & Vert, M. (2000). Selective enzymatic degradations of poly (L-lactide) and poly (ε-caprolactone) blend films. Biomacromolecules, 1(3), 350-359. [Google Scholar] [CrossRef].
Lu, H., Diaz, D. J., Czarnecki, N. J., Zhu, C., Kim, W., Shroff, R., … & Alper, H. S. (2022). Machine learning-aided engineering of hydrolases for PET depolymerization. Nature, 604(7907), 662-667. [Google Scholar] [CrossRef].
Mann, G. S., Singh, L. P., Kumar, P., Singh, S., & Prakash, C. (2021). On briefing the surface modifications of polylactic acid: A scope for betterment of biomedical structures. Journal of Thermoplastic Composite Materials, 34(7), 977-1005. [Google Scholar] [CrossRef].
Masaki, K., Kamini, N. R., Ikeda, H., & Iefuji, H. (2005). Cutinase-like enzyme from the yeast Cryptococcus sp. strain S-2 hydrolyzes polylactic acid and other biodegradable plastics. Applied and environmental microbiology, 71(11), 7548-7550. [Google Scholar] [CrossRef].
Mazhandu, Z. S., & Muzenda, E. (2019, November). Global plastic waste pollution challenges and management. In 2019 7th International Renewable and Sustainable Energy Conference (IRSEC) (pp. 1-8). IEEE. [Google Scholar] [CrossRef].
Mohanan, N., Montazer, Z., Sharma, P. K., & Levin, D. B. (2020). Microbial and enzymatic degradation of synthetic plastics. Frontiers in Microbiology, 11, 580709. [Google Scholar] [CrossRef].
Munim, S. A., & Raza, Z. A. (2019). Poly (lactic acid) based hydrogels: Formation, characteristics and biomedical applications. Journal of Porous Materials, 26(3), 881-901. [Google Scholar] [CrossRef].
Muthusamy, M. S., & Pramasivam, S. (2019). Bioplastics–an eco-friendly alternative to petrochemical plastics. Current world environment, 14(1), 49. [Google Scholar] [CrossRef].
Myburgh, M. W., Favaro, L., van Zyl, W. H., & Viljoen-Bloom, M. (2023). Engineered yeast for the efficient hydrolysis of polylactic acid. Bioresource Technology, 378, 129008. [Google Scholar] [CrossRef].
NatureWorks (2020). NatureWorks partners with Nonwovens Institute to support production of 10 million N95 masks for healthcare workers fighting COVID-19. Retrieve on Apr 3, 2022. [Website].
Nordin, N. M., Buys, Y. F., Anuar, H., Ani, M. H., & Pang, M. M. (2019). Development of conductive polymer composites from PLA/TPU blends filled with graphene nanoplatelets. Materials Today: Proceedings, 17, 500-507. [Google Scholar] [CrossRef].
Oda, Y., Yonetsu, A., Urakami, T., & Tonomura, K. (2000). Degradation of polylactide by commercial proteases. Journal of Polymers and the Environment, 8, 29-32. [Google Scholar] [CrossRef].
Princi, E. (2019). Rubber: science and technology. Walter de Gruyter GmbH & Co KG. [Google Scholar]
Qi, X., Ren, Y., & Wang, X. (2017). New advances in the biodegradation of Poly (lactic) acid. International Biodeterioration & Biodegradation, 117, 215-223. [Google Scholar] [CrossRef].
Reddy, N., Nama, D., & Yang, Y. (2008). Polylactic acid/polypropylene polyblend fibers for better resistance to degradation. Polymer Degradation and Stability, 93(1), 233-241. [Google Scholar] [CrossRef].
Rezvani Ghomi, E., Khosravi, F., Saedi Ardahaei, A., Dai, Y., Neisiany, R. E., Foroughi, F., … & Ramakrishna, S. (2021). The life cycle assessment for polylactic acid (PLA) to make it a low-carbon material. Polymers, 13(11), 1854. [Google Scholar] [CrossRef].
Rivero, C.P., Hu, Y., Kwan, T.H., Webb, C., Theodoropoulos, C., Daoud, W., & Lin, C.S.K. (2017). Bioplastics From Solid Waste. In Current Developments in Biotechnology and Bioengineering; Elsevier, 2017; pp. 1–26. [CrossRef].
Rodolfo, M. G., Costa, L. C., & Marini, J. (2022). Toughened poly (lactic acid)/thermoplastic polyurethane uncompatibilized blends. Journal of Polymer Engineering, 42(3), 214-222. [Google Scholar] [CrossRef].
Shuaib, M., Haleem, A., Kumar, L., Rohan, & Sharma, D. (2019). Design and analysis of steering knuckle joint. In Advances in Engineering Design: Select Proceedings of FLAME 2018 (pp. 423-431). Singapore: Springer Singapore. [Google Scholar] [CrossRef].
Sikder, P., Ren, Y., & Bhaduri, S. B. (2019). Synthesis and evaluation of protective poly (lactic acid) and fluorine-doped hydroxyapatite–based composite coatings on AZ31 magnesium alloy. Journal of Materials Research, 34(22), 3766-3776. [Google Scholar] [CrossRef].
Singhvi, M. S., Zinjarde, S. S., & Gokhale, D. V. (2019). Polylactic acid: Synthesis and biomedical applications. Journal of applied microbiology, 127(6), 1612-1626. [Google Scholar] [CrossRef].
Sourkouni, G., Jeremić, S., Kalogirou, C., Höfft, O., Nenadovic, M., Jankovic, V., … & Argirusis, C. (2023). Study of PLA pre-treatment, enzymatic and model-compost degradation, and valorization of degradation products to bacterial nanocellulose. World Journal of Microbiology and Biotechnology, 39(6), 161. [Google Scholar] [CrossRef].
Spierling, S., Knüpffer, E., Behnsen, H., Mudersbach, M., Krieg, H., Springer, S., … & Endres, H. J. (2018). Bio-based plastics-A review of environmental, social and economic impact assessments. Journal of Cleaner Production, 185, 476-491. [Google Scholar] [CrossRef].
Sukkhum, S., & Kitpreechavanich, V. (2011). New insight into biodegradation of poly (l-lactide), enzyme production and characterization. In Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications. IntechOpen. [Google Scholar] [CrossRef].
Sun, C., Wei, S., Tan, H., Huang, Y., & Zhang, Y. (2022). Progress in upcycling polylactic acid waste as an alternative carbon source: A review. Chemical Engineering Journal, 446, 136881. [Google Scholar] [CrossRef].
Szefer, E., Stafin, K., Leszczyńska, A., Zając, P., Hebda, E., Raftopoulos, K. N., & Pielichowski, K. (2019). Morphology, dynamics, and order development in a thermoplastic polyurethane with melt blended POSS. Journal of Polymer Science Part B: Polymer Physics, 57(17), 1133-1142. [Google Scholar] [CrossRef].
Teixeira, S., Eblagon, K. M., Miranda, F., R. Pereira, M. F., & Figueiredo, J. L. (2021). Towards controlled degradation of poly (lactic) acid in technical applications. C, 7(2), 42. [Google Scholar] [CrossRef].
Thushari, G. G. N., & Senevirathna, J. D. M. (2020). Plastic pollution in the marine environment. Heliyon, 6(8). [Google Scholar] [CrossRef].
Tokiwa, Y., & Calabia, B. P. (2006). Biodegradability and biodegradation of poly (lactide). Applied microbiology and biotechnology, 72, 244-251. [Google Scholar] [CrossRef].
Tsuji, H., & Miyauchi, S. (2001). Enzymatic hydrolysis of poly (lactide) s: effects of molecular weight, L-lactide content, and enantiomeric and diastereoisomeric polymer blending. Biomacromolecules, 2(2), 597-604. doi:. [Google Scholar] [CrossRef].
Williams, D. F. (1981). Enzymic hydrolysis of polylactic acid. Engineering in Medicine, 10(1), 5-7. doi:10.1243/EMED_JOUR_1981_010_004_02. [Google Scholar] [CrossRef].
Xiang, D., Zhang, X., Li, Y., Harkin-Jones, E., Zheng, Y., Wang, L., … & Wang, P. (2019). Enhanced performance of 3D printed highly elastic strain sensors of carbon nanotube/thermoplastic polyurethane nanocomposites via non-covalent interactions. Composites Part B: Engineering, 176, 107250. [Google Scholar] [CrossRef].
Xu, B., Chen, Y., He, J., Cao, S., Liu, J., Xue, R., … & Jiang, M. (2022). New insights into the biodegradation of polylactic acid: from degradation to upcycling. Environmental Reviews, 30(1), 30-38. [Google Scholar] [CrossRef].
Yuan, Q., Sun, R., Yu, P., Cheng, Y., Wu, W., Bao, J., & Alvarez, P. J. (2022). UV-aging of microplastics increases proximal ARG donor-recipient adsorption and leaching of chemicals that synergistically enhance antibiotic resistance propagation. Journal of Hazardous Materials, 427, 127895. doi:10.1016/j.jhazmat.2021.127895. [Google Scholar] [CrossRef].
Yuan, S., Shen, F., Chua, C. K., & Zhou, K. (2019). Polymeric composites for powder-based additive manufacturing: Materials and applications. Progress in Polymer Science, 91, 141-168. [Google Scholar] [CrossRef].
Zaaba, N. F., & Jaafar, M. (2020). A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polymer Engineering & Science, 60(9), 2061-2075. [Google Scholar] [CrossRef].
Zhou, Y., Luo, L., Liu, W., Zeng, G., & Chen, Y. (2015). Preparation and characteristic of PC/PLA/TPU blends by reactive extrusion. Advances in Materials Science and Engineering, 2015. [Google Scholar] [CrossRef].
About this Article
Cite this Article
APA
Ovinuchi E. (2023). Optimization of the Degradation Processes for Polylactic Acid using Microbial Enzymes: A Brief Summary. SustainE. 1(2), 1-14. doi:10.55366/suse.v1i2.1
Chicago
Ovinuchi Ejiohuo. “Microplastics: Environmental Impacts, Detection Techniques, and Mitigation Strategies.” SustainE 1, no. 2 (December 1, 2023): 1–24. https://doi.org/10.55366/suse.v1i2.1.
Received
6 September, 2023
Accepted
16, November 2023
Published
1 December 2023
Corresponding Author Email: ovinuchi.ejiohuo@gmail.com
Disclaimer: The opinions and statements expressed in this article are the authors’ sole responsibility and do not necessarily reflect the viewpoints of their affiliated organizations, the publisher, the hosted journal, the editors, or the reviewers. Furthermore, any product evaluated in this article or claims made by its manufacturer are not guaranteed or endorsed by the publisher.
Distributed under Creative Commons CC-BY 4.0
Share this article
Use the buttons below to share the article on desired platforms.